Coronary heart disease (CHD) remains the leading cause of death in Western civilizations. The role of abnormal lipid metabolism as a modifiable risk factor for CHD is well documented. The presence of chronic kidney disease (CKD) is also associated with an increased risk of CHD, but the pathogenesis of this relationship is not completely understood. Specifically, it remains unclear whether and to what degree the management of dyslipidemia can affect the risk of CHD in patients with advanced CKD, especially given the higher risk of adverse effects with pharmacological therapy in this patient population.
Epidemiology
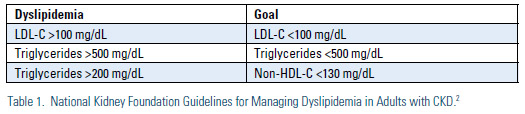
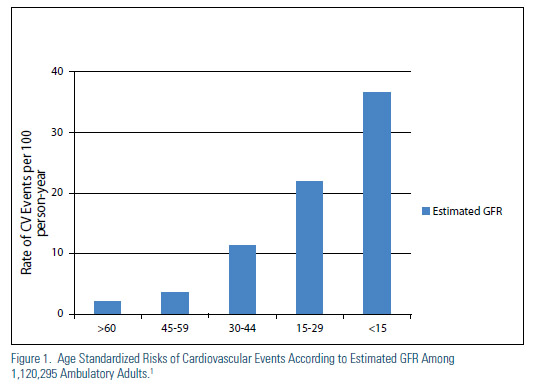
Decreased kidney function is associated with an increase in the risk of cardiovascular (CV) death, particularly among those with stage IV and V CKD (Figure 1).1 Relative to the general population, advanced CKD does appear to be associated with an increase in triglycerides and VLDL-C and a decrease in HDL-C, but LDL-C appears to be relatively unchanged or perhaps a bit decreased. The epidemiological data has led many to suggest that advanced CKD should be considered a CHD risk equivalent. Indeed, the National Kidney Foundation has published guidelines for management of lipids in CKD that set aggressive goals of therapy, similar to those defined by ATP III for other CHD risk equivalents (Table 1).2
Pharmocokinetics, Pharmacodynamics and Potential for Drug Interactions
Statins and other lipid-lowering agents have been used clinically for the management of hyperlipidemia and prevention of cardiovascular events in patients with CKD for the past 25 years. Statins with a shorter duration of action should be dosed at night or bedtime since cholesterol biosynthesis undergoes a circadian cycle, with most cholesterol formation occurring while an individual is asleep. The ideal antihyperlipidemic agent should improve patients’ lipid profile without increasing the risk of toxicity. The comparison of pharmacokinetics properties of all antihyperlipidemics agents are listed in Table 2.3 Most statins rely on both renal excretion and hepatic metabolism for elimination; atorvastatin and fluvastatin rely least on renal excretion. Most statins are metabolized through cytochrome p450-IIIA4 and can result in significant drug interactions. Compared to other statins, pravastatin and pitavastatin are more hydrophilic agents and metabolized through hydroxylation and glucuronide conjugation, respectively. In general, statins should be used with caution in patients with CKD, particularly the elderly population with CKD. Most patients with CKD have a number of comorbid conditions that can mask early signs and symptoms of myopathy and rhabdomyolysis [osteoarthritis and back pain] or place these patients at greater risk of drug-drug interactions. Recently, the U.S. Food and Drug Administration (FDA) issued recommendations concerning drug-drug interactions for both lovastatin and simvastatin.4
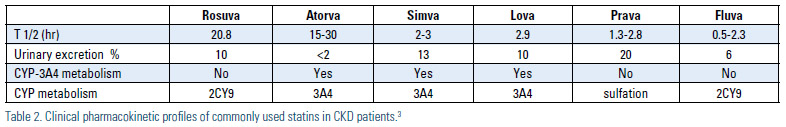
For example, the use of cyclosporine and simvastain/ lovastatin are relatively contraindicated, while pravastatin is considered the safer agent in this setting. The most serious form of myopathy, rhabdomyolysis, is more common in CKD and can be fatal. Gemfibrozil may affect oxidation of statins or act as an inhibitor of the P450 enzyme system, thereby increasing the area under the curve and total drug exposure of most statins, which may explain the high incidence of myopathy observed with this combination.5 The use of gemfibrozil and statins is considered a relative contraindication. If a fibric acid derivative is needed, fenofibrate is the preferred agent, but dose adjustments are required in patients with CKD. Fenofibrate may increase creatinine production and cause increases in serum creatinine values. In the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, a lower estimated glomerular filtration rate (eGFR), creatinine clearance and higher serum creatinine was reported in the fenofibrate group compared to the control group. However, the elevation of serum creatinine and cystatin C was noted without any changes in tubular function.6
Clinical Endpoints in CKD
While treatment of dyslipidemia has been demonstrated to decrease the incidence of CV events in both primary and secondary prevention, the majority of our landmark prospective clinical trials of lipid-lowering therapy either excluded or enrolled few patients with advanced CKD. As CKD advances the relationship between dyslipidemia and CV risk becomes less clear. Indeed, it has been suggested that the pathophysiology of CV events may be different in patients with more advanced CKD, whereby many CV events could be caused by vascular stiffness and calcification, structural heart disease, increased sympathetic tone, arrhythmia and transient decreases in perfusion across fixed areas of stenosis rather than atherothrombotic plaque rupture.
There have been only three large-scale, prospective, randomized trials of lipid lowering therapy in patients with advanced CKD examining the incidence of clinical CV events (Figure 3). In the first two of these studies, the Die Deutsche Diabetes Dialyze Studie (4D) and the AURORA study, the use of high potency statins (atorvastatin and rosuvastatin respectively) dialysis patients did not lead to a significant reduction in cardiovascular events despite robust changes in lipid parameters.7,8
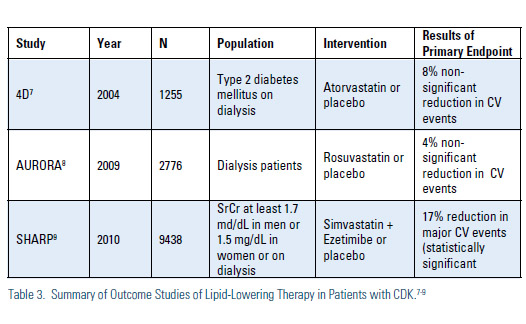
In the SHARP study, the use of simvastatinezetimibe in subjects with stage 3-5 CKD did lead to a statistically significant 17% reduction in CVD events.9 In subgroup analysis of SHARP, subjects with stage 3 CKD had a statistically significant 25% reduction in CV events; those with stage 4 CKD had a statistically significant 22% reduction in CV events; while those on hemodialysis at baseline did not have a significant reduction in events. Whether the favorable results seen in SHARP were due to the specific combination of lipidlowering agents used in that study or the inclusion of patients with less advanced CKD (as seems more likely) is unknown. Importantly, although other combinations of lipid-lowering agents, including statin + nicotinic agent or statin + fibrate, are frequently employed in patients with advanced CKD, endpoint data with these combinations is essentially non-existent in this patient population.
A recent meta-analysis of all trials of lipidlowering therapy that included patients with CKD suggested that lipid-lowering therapy led to a modest decrease in the risk of cardiac mortality (pooled risk ratio [RR] from 6 trials 0.82), CV events (pooled RR from 9 trials 0.78), and myocardial infarction (pooled RR from 9 trials 0.74); however, the majority of subjects in the included studies were stage 3 CKD patients, representing subgroups of larger studies.6,10 A second meta-analysis demonstrated that statin therapy reduced all cause and CV mortality, major CV events, myocardial infarction and stroke in person with CKD not receiving dialysis by about 20-25%, but that there was little or no beneficial effect on any mortality or CV endpoints with statin therapy in those on dialysis at baseline.11 Unfortunately, in both meta-analyses, most patients with CKD not on dialysis were stage 3 or lower, and the data was not reported for stage 4 CKD individually.
There are very limited clinical data on the safety and efficacy of combination of statin and nicotinic acid or statin and fibrates therapies in patients with CKD. Due to possible drug interactions and risk of myopathy and recent findings from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study and AIMHIGH (Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes, that combination therapy did not result in a significant reduction of cardiovascular events compared with statin monotherapy, care should be taken when combination therapy with these agents is utilized with statins in patients with chronic kidney disease.12, 13
While lipid-lowering therapy appears effective in reducing CV events in stage 3 CKD, there is little to no evidence that lipid-lowering therapy reduces CV events in patients already on dialysis. Data specifically in stage 4 patients is quite limited, but based on the SHARP study results, these patients probably do receive a modest benefit.
Safety of Lipid-Lowering Agents in Advanced CKD
In general, cumulative data from both primary and secondary prevention studies of statins indicate that the HMG CoA reductase inhibitors (statins) have an excellent safety record and a favorable riskbenefit profile, with a low risk of significant adverse events (<1% incidence).14 However, epidemiological studies indicate the discontinuation rates for lovastatin and simvastatin were 3% and 6% respectively, and much higher incidence of myopathy at high doses (80 mg daily).15
Myopathy–The clinical spectrum of statin-induced myopathy consists of myalgia, myositis, rhabdomyolysis and asymptomatic increases in plasma creatine kinase (CK) levels. Muscle-related adverse events can be difficult to describe because the terminology used can be inconsistent. Generally, 'myalgia' refers to the tolerability issue of muscle pain or weakness without elevation of creatine kinase (CK) levels, while 'myositis' is defined as the safety issue of muscle pain with considerable elevation of CK levels.16 Rhabdomyolysis refers to muscle symptoms with markedly raised CK, usually >10x upper limit of normal, and is generally considered a serious medical condition that can lead to hospitalization, renal failure, and even death.16 For all statins, the overall risk of rhabdomyolysis is less than 0.5% in the general population.17 This risk may be higher in patients with CKD, elderly, and in patients taking other drugs or food which inhibit CYP3A4, specifically grapefruit, cyclosporine, azole antifungals, macrolide antibiotics, and fibrates.18 Although the exact pathogenesis of myopathy has not been determined, several mechanisms have been postulated. Myopathy may be due to mitochondrial dysfunction and muscle protein degradation. The genetic marker SLCO1B1 is among the strongest predictors of myopathy risk.19
Hepatotoxicity–Previously, hepatocellular necrosis and hepatotoxicity induced by statins were considered a myth.20 A recent study has concluded that idiosyncratic hepatotoxicity may be associated with the use of statins.21 Asymptomatic hepatic transaminase elevation (greater than three times the upper limit of normal) may occur in 1-2% of patients on an HMGCoA reductase inhibitor and in general is dose related. In most patients, elevation of transaminase enzymes are resolved spontaneously with continued therapy, although discontinuation may be required in some patients. Based on current recommendations from the FDA, routine monitoring of transaminases is no longer necessary unless the patient exhibits transaminase abnormalities at baseline, has other risk factors for hepatotoxicity, or clinically abnormalities are suspected.
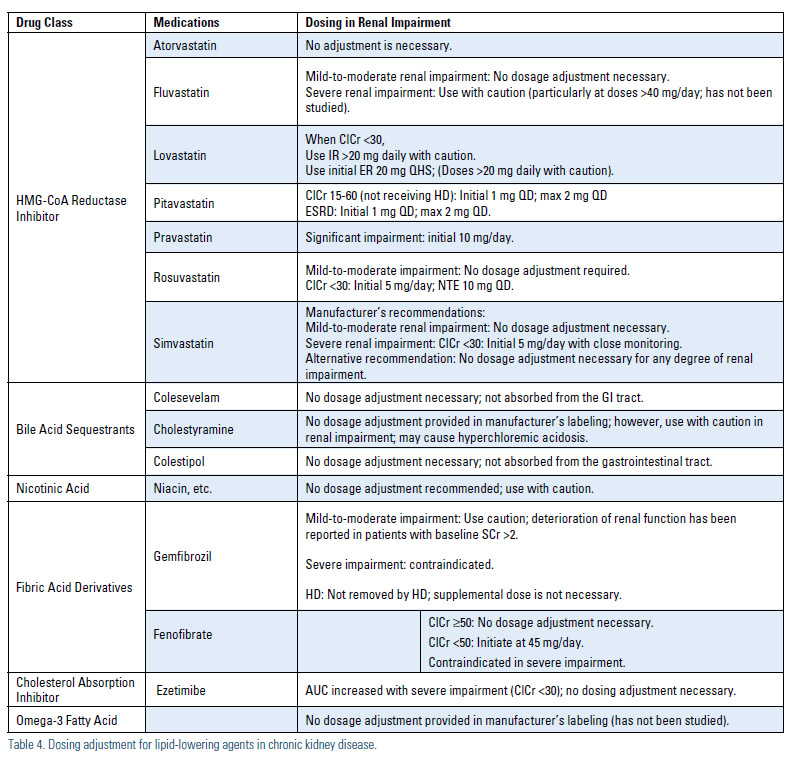
Dosing–With the increasing incidence of chronic renal disease, regular renal function monitoring and dosage adjustment of lipid-lowering agents according to eGFR and pharmacokinetic data are of major importance.3,22 Because large studies of the safety of these agents in patients with CKD is lacking, drug-drug interactions and dosage adjustment recommendations need to be regularly updated following the results of epidemiological and observational studies. Patients with significant renal disease should be started on low doses of statins and other lipid-lowering agents, with doses titrated up only when clinically indicated. Due to the risk of significant drug-drug interactions, for the renal transplant population fluvastatin has been demonstrated to be safe in a large clinical endpoint trial where tacrolimus was the most common immunosuppressant used, but pravastatin may be a more suitable agent when cyclosporine is used (Table 4).23
Summary
While far from conclusive, the available clinical trail evidence supports the hypothesis that lipid-lowering therapy may provide diminishing benefit as CKD advances. In stage 4 CKD and dialysis, the potential for benefit from statin therapy needs to be weighed carefully against the increased risk for adverse effects seen in this patient population. With the exception of statin-ezetimibe, combination lipidlowering therapy has not been well studied in this patient population and should be used only with careful monitoring for adverse events. Future studies are needed to help clinicians appropriately balance the benefits and risks of lipid-lowering therapy in CKD, particularly for the increasing number of patients with Stage IV CKD and concomitant dyslipidemia.
Disclosure statement: Dr. Bloch has received honoraria from Aegerion, AstraZeneca, Chelsea Therapeutics, Daiichi Sankyo Inc., LipoScience Inc., PriMed CME, and Takeda Pharmaceuticals. Dr. Olyaei has no disclosures to report.






.jpg)
.png)












