Over 26 million Americans have chronic kidney disease (CKD). At risk for CKD include 65 million Americans with hypertension and 20 million patients with diabetes mellitus. The risk of cardiovascular disease (CVD), including coronary, cerebrovascular, peripheral vascular disease, and congestive heart failure increases by 3-to-20-fold as CKD progresses.
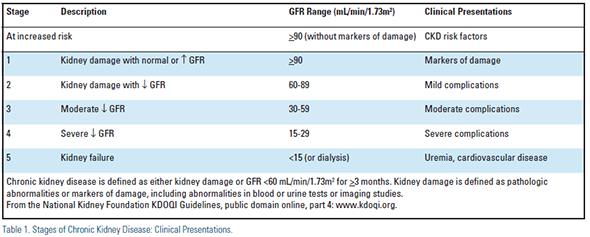
The staging of CKD, and slowing (or potentially halting) the progression of CKD through its five stages (Table 1) are paramount, but require recognition of CKD from the outset.1-3
There is a developing epidemic of obesity, diabetes mellitus, metabolic syndrome, aging of the population, and an associated increasing prevalence of CKD and CVD of enormous proportions and costs which are unsustainable.
The kidneys receive 25% of cardiac output and are a major vascular organ. Risk factors for CVD and CKD are similar and include hypertension, dyslipidemia, diabetes mellitus, tobacco abuse, advancing age, obesity, physical inactivity, positive family history, and ethnicity. Treatment modalities that benefit patients with normal renal function have not provided the expected CV event reduction in CKD, as other metabolic and inflammatory factors are inherent in CKD patients1 including:
- Reduced glomerular filtration rate (GFR) which can be present despite apparently normal serum creatinine levels
- Elevated Cystatin C
- Elevated microalbuminuria or proteinuria
- Renin-angiotensin system over activity
- Left ventricular hypertrophy
- Anemia
- Abnormal calcium and phosphorus metabolism, which are linked to bone disease and vascular calcification
- Elevated lipoprotein(a) and homocysteine
- Hypovitaminosis D
- Thrombogenic factors
- Oxidative stress and inflammation
Patients with CKD usually die of CVD. The National Kidney Foundation considers CKD a coronary risk equivalent, and that all patients with CKD, especially stages 3-5, should be considered in the highest risk group irrespective of traditional CVD risk factors4 (see NKF Kidney Disease Outcome Quality Initiative, at www.kdoqi.org). However, early CKD is often not recognized, and there often is "therapeutic nihilism" in patients with CKD, including under-use of aspirin, statins, beta blockers, and suboptimal blockade of the reninangiotensin system, even in the setting of acute coronary syndrome or CHF.
Only 35% of patients who are within 1 to 6 months of requiring renal replacement therapy for end stage renal disease (dialysis) have ever received a nephrology consultation. Patients referred late (<4 months before ESRD) have a 72% greater mortality during the first year.4
How to identify CKD1,5
- Measure serum creatinine and calculate estimated GFR (eGFR) using the modified Modification of Diet in Renal Disease (MDRD) equation that can be performed quickly at www. kdoqi.org.in all patients at risk for CKD, especially patients with known CVD, diabetes mellitus, hypertension, dyslipidemia, the elderly, or in those with a family history of kidney disease.1 Calculated eGFR using this formula is very accurate for levels <60 ml/min/1.73m2 and requires only age, sex, race and serum creatinine as pertinent variables. An eGFR <60 (stage 3 CKD) is associated with an increased risk for CVD and accelerated decline of renal function. Serum creatinine may appear "normal," particularly in older patients and women of Caucasian ethnicity who may have decreased muscle mass, a normal creatinine but an abnormal eGFR.
- Random ("spot") urine sample for albumin/creatinine ratio (abnormal: >30 mg albumin/g creatinine).
A newer and apparently more accurate assessment of renal dysfunction and likely presence of microalbuminuria and a potential CVD risk indicator is derived from measurement of Cystatin C. Cystatin C is a cysteine protease inhibitor that is produced by all nucleated cells, filtered by the kidney, and degraded by the proximal tubule. Its levels are not dependent on muscle mass.6
Microalbuminuria (> 30-300 mg albumin/g creatinine) is strongly associated with endothelial damage and susceptibility to atherogenesis.; and its presence more than doubles the predicted risk of atherosclerotic disease, especially in patients with hypertension and diabetes mellitus.
Microalbuminuria or macroalbuminuria (clinical proteinuria >300 mg/g creatinine) is associated with progression of CKD and CVD, and is also associated with dyslipidemia and elevated lipoprotein(a), even without a reduction in eGFR.
Renin angiotensin system blockade with an angiotensin converting enzyme inhibitor (ACEI) and/or or an angiotensin receptor blocker (ARB) reduces proteinuria and may reduce progression of renal disease, but has not yet been shown to consistently reduce CVD events.7
The renin inhibitor aliskerin should not be used in combination with an ACE inhibitor or ARB in patients with diabetes or in patients with moderate to severe renal impairment. The ALTITUDE trial which studied aliskerin was stopped because of an increased risk of fatal strokes, acute renal failure, hyperkalemia, and hypotension in the active arm. (Drug Safety Communication, www.fda.gov, posted on 4/20/2012).
Oxidative Stress and inflammation
Oxidative stress can provoke inflammation by activating transcription factor nuclear factor-kappa B (NF-kB), the master regulator of proinflammatory cytokines, chemotactic and profibrotic factors. Oxidative stress also creates oxidized lowdensity lipoprotein and advanced glycation end products and oxidized phospholipids, all of which are extremely pro-oxidant and pro-inflammatory.1
Increased activation of the reninangiotensin system in renal tissue, irrespective of systemic levels, leads to formation of reactive oxygen species (ROS). That is mechanistically why blocking this system may potentially retard progression of CKD.
Retarding Progression of CKD and Reducing CV Risk (for complete discussion, see www.kdoqi.org and references) The staging of CKD is shown in Table 1. Seek specialty consultation with a nephrologist for stage 3 CKD and microalbuminuria!
Lifestyle modifications are essential for controlling CVD risk factors. Dietary salt restriction is vital, because dietary sodium excess reduces the effectiveness of antihypertensive drugs and treatment of proteinuria. A lower protein diet and low phosphate diet are important in patients with proteinuria and calcium-phosphate imbalance.
Control of hypertension is critical. Often with advanced CKD (especially dialysis patients) we find underuse of beta blockers. This is unfortunate because CKD patients on dialysis often die of arrhythmias or heart failure. Inhibiting the renin-angiotensin-aldosterone system with ACE inhibition or angiotensin receptor blockade may be renoprotective, and their anti-proteinuric effects go beyond lowering of BP.
Do not withhold these agents solely on the basis of renal function! This deprives patients of potential CVD prevention benefits. A modest (20-25%) increase in serum creatinine is to be expected with the use of an ACE inhibitor or ARB. Modest dietary potassium restriction usually is adequate to maintain normal serum potassium when ACE or ARBs are used.
Control of dyslipidemia is often overlooked or substandard in patients with CKD. Lipoprotein changes are very similar to those seen in the metabolic syndrome. They include elevated triglycerides and atherogenic remnants, low HDL C, increased small LDL particles, and elevated lipoprotein(a). While LDL-C may not be exceptionally elevated, the number of LDL particles invariably is. In this population, we believe that non- HDL-C (total cholesterol minus HDL-C) better predicts CVD risk than LDL-C. ATP III supports this as a secondary goal for therapy when triglycerides are >200 mg/dL. LDL-C target level is <100 mg/dL (non-HDL-C < 130 mg/dL) in patients with CKD, and < 70 mg/dL (non-HDL-C < 100 mg/dL) in renal patients with established CVD.1,8,9 LDL particle number by NMR Lipoprofile, or an apoB level, may improve risk prediction and determination of therapeutic goals and should be considered.
Statins appear to be reno-protective and may reduce proteinuria. They are an essential component of lipid-lowering therapy to reduce atherosclerotic risk present in patients with CKD.
Statins appeared to reduce CV events in the few patients with stage 3 and 4 CKD in early trials (however please note that such patients were often excluded from these trials).8 Reduction in mortality with statins has not been shown in patients on dialysis (stage 5) likely due to the high death rate from arrhythmias and heart failure and sudden cardiac death in patients with stage 5 CKD, which likely masks any benefit of reduced myocardial infarction when statins are used.
The recent SHARP trial showed clear benefit in CVD risk reduction in patients with advanced CKD using simvastatin/ ezetimibe therapy. There was a trend toward benefit in stage 5-dialysis patients. This large trial looked at 9270 patients with predominantly stage 3 and 4 CKD, focusing on patients with eGFR below 60 and especially between 15 and 45. The combination low dose simvastatin and ezetimibe reduced CV events after a median of 4.9 years follow-up. There was no effect on progression of CKD. There were very few side effects of therapy reported.10
Control of diabetes mellitus reduces progression of CKD. Strict glycemic control prevents or delays the onset of diabetic kidney disease. Data is inconclusive with regard to progression of established disease. We believe that control of BP and lipids is of paramount importance in these patients.
Smoking cessation reduces the rate of progression of CKD.
Treatment of Lipoprotein Abnormalities in CKD Statins.1,8,9 Even with fairly normal LDL-C in many CKD patients (and one can argue for an on treatment LCL-C < 70 mg/dL), apoB or LDL-P will almost certainly be significantly elevated and the usually elevated triglyceride concentrations imply an elevated apoB or elevated LDLP. The safest statins appear to be the ones requiring the least renal clearance; these are atorvastatin, simvastatin and fluvastatin, especially for an EGFR < 30. Maximum doses should not be used due to a higher risk of myopathy in these patients.
Fibrates may lower elevated triglycerides and raise HDL-C, but have dose limitations because they are metabolized and cleared by the kidney. Gemfibrozil theoretically would be the fibrate of choice to treat high triglycerides as it is less affected by renal clearance but there is a higher risk of muscle injury with this agent in combination with a statin. It would seem prudent to restrict the dose to 600 mg once a day with an eGFR < 60, and to avoid its use with an eGFR < 15.
Fenofibrates pose less of a risk of myopathy in combination with a statin, and dose reduction of 50% is recommended when eGFR is between 60-90 and 75% with eGFR between 15 and 60. Fibrates should not be used with eGFR < 15. Fenofibrate use may reduce microalbuminuria in diabetic patients. Fibrates do not lower apoB or LDL-P, however. Fibrates may raise serum creatinine by a non-renal mechanism as well, which is reversible but can be confusing.
Niacin may have a special role in CKD despite tolerability issues, since it is an effective drug to lower apoB/LD-P, lower high triglycerides, and raise HDL-C. Niacin has an additional advantage in reducing intestinal phosphate absorption, which is not well known. Renal clearance is about 35%. Doses up to 1500 mg a day appear safe.
Ezetimibe is safe to use at all levels of eGFR. One very much underappreciated aspect of lipoprotein metabolism is cholesterol and sterol ester hyperabsorption when liver synthesis of cholesterol is reduced, as by a statin or niacin. Sterol esters may be quite atherogenic. With hyperabsorption, ezetimibe would be the best option to add to further lower apoB/LDP-P, and then the dose of the statin could likely be lowered.
Sevelamer, used to treat hyperphosphatemia in more advanced CKD, behaves like a bile acid binding resin to lower apoB/LDL-P. Colesevelam can be used as a bile acid binding drug to lower apoB/LDP. These agents may raise triglycerides but may be of use especially when intolerance to statin or niacin therapy is encountered.
Omega-3 fatty acid fish oil therapy may offer benefits to lower very high triglycerides.
Of the above mentioned therapies, only statins have clinical trial evidence for reduced CVD events in CKD.
Key issues and Summary
Suspect CKD in any patient with CVD risk factors. Determine eGFR and check for microalbuminuria in patients at risk for CVD.
The presence of CKD should be considered a coronary risk equivalent, with the need for a global CVD risk reduction program. Treatment of dyslipidemia is paramount generally with a statin, and other therapy as needed to deal with mixed dyslipidemia often found in patients with CKD.
Proteinuria is a marker for CKD; it contributes to the progression of CKD, and it is an independent predictor of increased risk for CVD and death in patients with CKD.
An angiotensin converting enzyme inhibitor or an angiotensin receptor blocker should be strongly considered for therapy in the face of CKD and/or significant proteinuria.
Patients with CKD and an eGFR below 60 mL/min1.73m2 nephrologists believe should be assessed for LVH, anemia, parathyroid, mineral, and vitamin D abnormalities, and lipoproteins including apoB or LDL-P, and insulin resistance.
Primary care physicians and nephrologists need to share responsibility for care of CKD patients. See detailed information from the National Kidney Foundation at www.kdoqi.org.
Disclosure statement: Dr. Ferguson has no disclosures to report.
References for this article are listed on Page 32 of Lipid Spin PDF available here.





.jpg)
.png)











