Statins currently stand as the cornerstone of therapeutic strategies for the reduction of atherosclerotic cardiovascular events.1–4 However, despite their chronicled successes over the past 30 years since the U.S. Food and Drug Administration (FDA) approved lovastatin, multiple analyses of clinical trial data reveal residual cardiovascular risk in patients treated with statins, even those achieving optimal reduction of low-density lipoprotein cholesterol (LDL-C).5 Therefore, the pharmaceutical industry has turned to new therapeutic modalities to modulate this residual risk in statin-treated patients.
A major area of focus over the past 10 years has been modulation of cholesteryl ester transfer protein (CETP). Interest in this target was based on several observations, including:
- The physiologic actions of CETP in increasing high-density lipoprotein- cholesterol (HDL-C)6
- The involvement of high-density lipoproteins (HDLs) in the reverse cholesterol transport pathway and the inverse association of high-density lipoprotein cholesterol (HDL-C) concentration with cardiovascular disease incidence7
- A link between genetic variants of CETP activity and the risk for coronary artery disease8,9
Pfizer developed and tested the first CETP inhibitor, torcetrapib. In the Phase 3 Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events (ILLUMINATE) trial, patients treated with torcetrapib had significantly higher rates of major cardiovascular and cerebrovascular events, and higher mortality resulting from cancer and infection.10 The trial, and all further development of torcetrapib, was terminated in December 2006. It is largely accepted that off-target effects on cortisol, endothelin-1, and aldosterone led to the observed adverse outcomes.11 Dalcetrapib, developed by Hoffman-La Roche, followed torcetrapib. In the Phase 3, Dalcetrapib in Stable Coronary Heart Disease Patients with Recent Acute Coronary Syndrome (dal-OUTCOMES) trial, treatment with dalcetrapib did not alter the risk of major cardiovascular events.12 The study was stopped early based on a futility analysis and Roche discontinued development of dalcetrapib in May 2012. In other studies, it was shown that dalcetrapib did not have any off-target adverse effects; however it did not have any appreciable effect on LDL-C, which was the major reason cited for the negative results. Eli Lilly recently halted development of evacetrapib in October 2015, after a planned interim analysis of its Phase 3 Aliskiren and the Calcium Channel Blocker Amlodipine Combination as an Initial Treatment Strategy for Hypertension (ACCELERATE) trial also demonstrated insufficient efficacy. Results of the study were presented at the 2016 American College of Cardiology Scientific Sessions and publication of the full results are expected this year. Evacetrapib had no off-target effects, decreased LDL-C by 37 percent, and increased HDL-C by 130 percent, yet did not decrease atherosclerotic cardiovascular events in more than 12,000 high-risk patients.13 No reasons for the negative results of evacetrapib have yet been hypothesized.
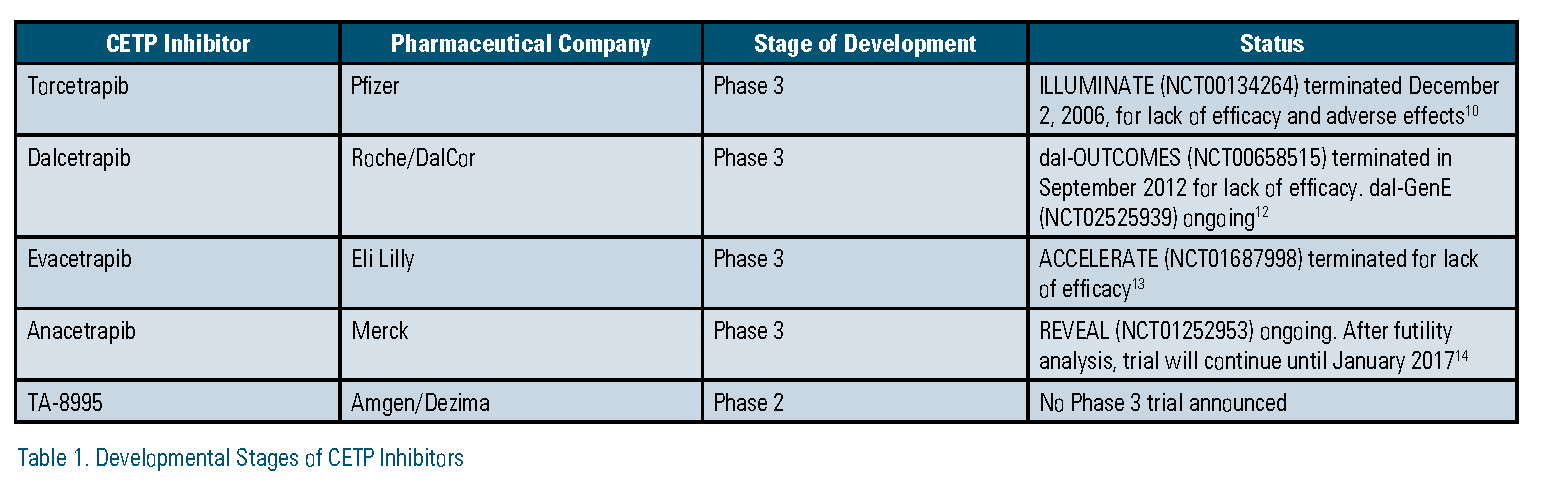
After three straight negative outcomes trials, significant questions exist about the validity of CETP as a therapeutic target for reducing residual risk. However, there are two CETP inhibitors that remain in different phases of development and one that has been resurrected based on pharmacogenomics data.
Following the announcement of the termination of evacetrapib, Merck added a futility analysis to its planned interim review of the Phase 3 Rapid Evaluation of Vessel Healing After Angioplasty (REVEAL) trial of its CETP inhibitor, anacetrapib. On Nov. 13, 2015, Merck announced that, based on this analysis, REVEAL would continue with no changes to its original planned completion date.14 In the months since this announcement, many have questioned the reasons underlying this decision given three past CETP inhibitor failures and Merck’s huge capital investment in anacetrapib. There are some reasons speculated as to why this decision was made. First, although anacetrapib has similar effects on LDL-C and HDL-C as evacetrapib, the REVEAL trial is more than twice as large as ACCELERATE, which increases the statistical power to detect a difference in associated outcomes. Second, anacetrapib reduces lipoprotein (a) (Lp[a]) by about 32 percent.15 This effect is thought to be shared by evacetrapib. However, at the time of this writing, no data for evacetrapib was available for comparison.16 Nevertheless, should REVEAL prove positive and anacetrapib make it to market, one area of concern is its unusually long terminal half-life. In a subset of 30 patients from its Phase 2 Determining the Efficacy and Tolerability of CETP Inhibition with Anacetrapib (DEFINE) trial, low concentrations of anacetrapib were detectable in plasma as late as four years after the last dose was taken.17 Using population pharmacokinetic modeling, the estimated half-life was 550 days. Although no accumulation of drug is expected, it remains what impact this would have in patients who suffer adverse drug reactions or who become or expect to become pregnant during treatment.18
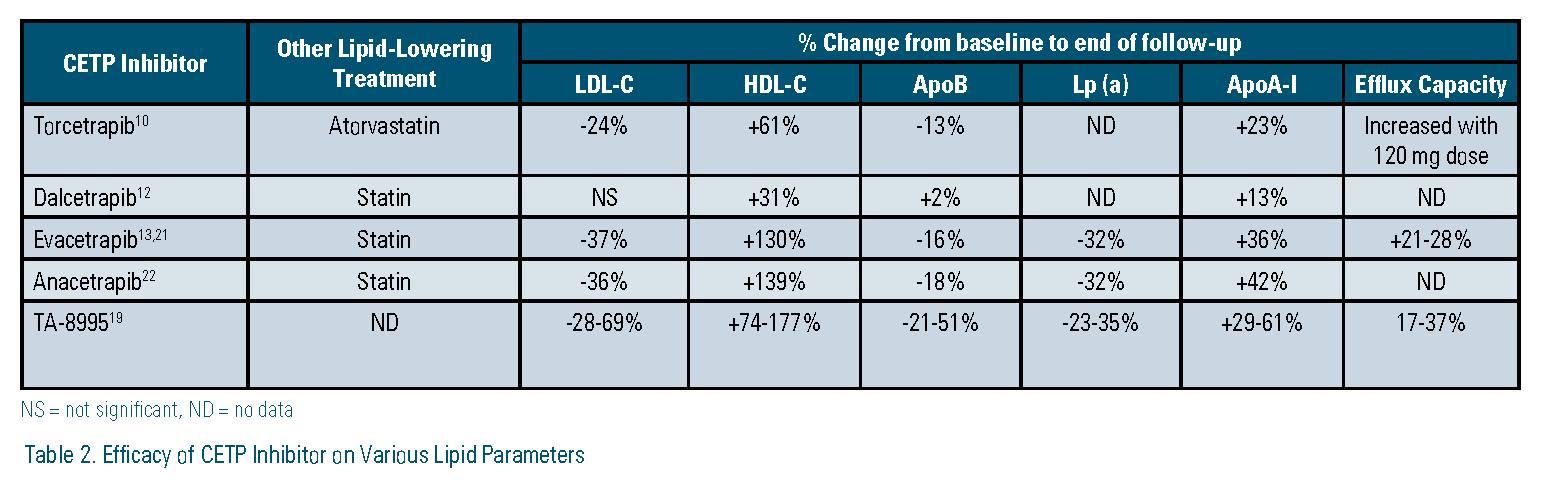
On Sept. 16, 2015, prior to Eli Lilly’s announcement about evacetrapib, Amgen acquired the oral CETP inhibitor TA-8995 in a deal to purchase Dezima Pharma, which had developed TA-8995 to that point. Data from its Phase 2 Cholesterol ester transfer protein inhibition by TA-8995 in Patients with Mild Dyslipidaemia (TULIP) trial identified several potentially advantageous properties. First, it decreased LDL-C up to 69 percent, increased HDL-C up to 177 percent, decreased apolipoprotein B (apo B) up to 51 percent, and decreased Lp (a) up to 35 percent, making it the most potent CETP inhibitor. Second, it decreased increased apolipoprotein A-I (apo AI) up to 63 percent and increased cholesterol efflux up to 37 percent, suggesting functional HDL particles. Third, it has a much shorter half-life — about two weeks compared to anacetrapib.19 There has been no announcement by Amgen as to whether TA-8995 will enter a Phase 3 clinical trial.
Following Roche’s termination of the development of dalcetrapib, investigators at the Montreal Heart Institute conducted a retrospective analysis of a cohort of patients from the dal-OUTCOMES and Safety and Efficacy of Dalcetrapib on Atherosclerotic Disease Using Novel Non-invasive Multimodality Imaging (dal- PLAQUE-2) studies. A single nucleotide polymorphism (SNP) identified at a specific location in the adenylate cyclase type 9 (ADCY9) gene on chromosome 16 was found to be associated with cardiovascular events in dalcetrapib- treated patients. In patients homozygous for the minor allele (AA), there was a 39 percent decrease in the trial’s composite endpoint, which was coronary heart disease death, resuscitated cardiac arrest, nonfatal myocardial infarction, nonfatal stroke, unstable angina, or urgent coronary revascularization. In patients homozygous for the wild-type allele (GG), there was a 27 percent increase in the same endpoint. No such genetic differences were observed in patients receiving placebo.20 This analysis was sponsored by Roche, which subsequently filed for patents covering this genetic marker for use with dalcetrapib and the other CETP inhibitors. A different pharmaceutical company, DalCor, acquired exclusive licensing rights from Roche for the use of dalcetrapib and the genetic marker. DalCor is sponsoring its own Phase 3 trial, the Effect of Dalcetrapib vs. Placebo on CV Risk in a Genetically Defined Population with Recent ACS (dal-GenE). The trial plans to enroll 5,000 patients who recently have been hospitalized with acute coronary syndrome and have a previously identified AA genotype of the ADCY9 gene. It started recruiting patients in March. There have been several criticisms of this trial including a lack of foundational knowledge about the functions of the ADCY9 gene and its link the mechanism of CETP.
In summary, of the four CETP inhibitors that have been brought to clinical trials, three have been abandoned due to lack of clinical benefit or off-target effects. Anacetrapib and TA-8995 remain in development. The REVEAL trial with anacetrapib is large enough that it may ultimately show cardiovascular benefit, yet it remains to be seen whether the benefit will be appreciable enough for clinical utility. TA-8995 showed some promising results in its phase II study; however, similar results were demonstrated with other CETP inhibitors that ultimately did not translate to improvements in outcomes of their phase III trials. Dalcetrapib has been resurrected based on observed benefit in a genetic subgroup of patients. While this shows promise for dalcetrapib and possibly the other CETP inhibitors as well, there are unknowns about the plausibility of the genetic link.
Disclosure statement: Dr. Stewart has no disclosures to report.
References are listed on page 45 of the PDF.






.jpg)
.png)











