Cholesterol is an essential component of human cell biology, responsible for numerous diverse and important physiologic functions. In addition to maintenance of cell membranes, cholesterol is a key substrate for production of vitamin D, and thyroid and steroid hormones. Previous studies hypothesized adrenal insufficiency may occur in individuals with LDL/HDL deficiency, although little data is available. Given its importance in maintaining proper cellular function and maintenance, vital systems such as the adrenals have evolved multiple pathways to assure an adequate supply of cholesterol for basal metabolism and response to stress.
Although the production of biologically active steroids by the adrenal cortex is under direct control of adrenocorticotropic hormone (ACTH), cholesterol availability is critical for optimum adrenal function. The acquisition of cholesterol for steroidogenesis comes from a variety of sources and involves multiple enzymes to maintain production of pregnenolone, the initial step in production of glucocorticoids, mineralocorticoids, and sex steroids. Four major sources of cholesterol have been identified (Figure 1). Numerous intracellular proteins are also required for proper trafficking of cholesterol to the outer mitochondrial membrane.
A variety of transport proteins (not shown) are involved in trafficking free cholesterol in the cytosol to the mitochondria. While all steroid producing cells are capable of de-novo synthesis of cholesterol, the adrenal preferentially utilizes cholesterol from LDL and HDL.(4) Cholesterol derived from uptake of LDL via LDL receptor-mediated endocytosis and other apolipoprotein B- or E-containing lipoprotein pathways appears to be one of two primarily preferred pathways for cholesterol availability in adrenal cells.
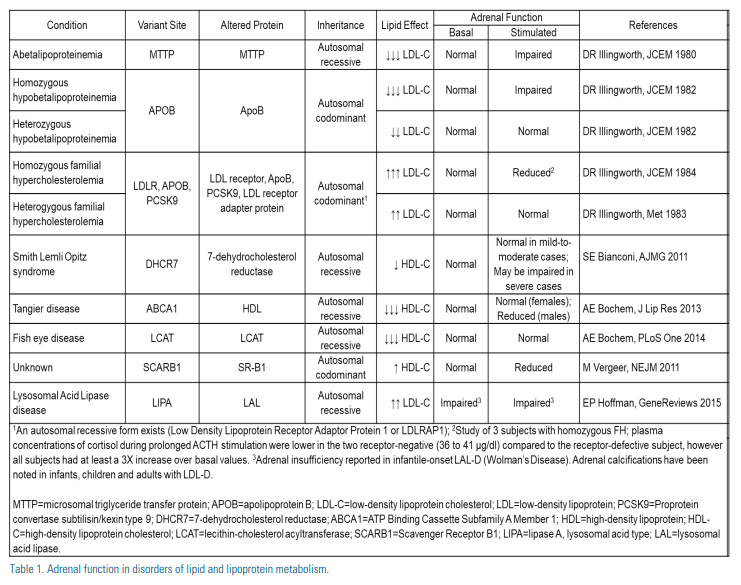
The importance of the LDL receptor pathway in adrenal cells is highlighted by the observation that individuals with absent or near absent circulating levels of LDL-C (i.e., homozygous hypobetalipoproteinemia and abetalipoproteinemia) have been reported to have impaired adrenal function (Table). (5, 6) It is unclear, however, if adrenal impairment in these conditions is due to lack of intracellular cholesterol – as alternate compensatory mechanisms exist – or the result of alteration of cellular structure/function in the setting of absent or near absent levels of circulating LDL-C. Support for the latter includes observations of those with low but not absent or near absent levels of circulating LDL-C (i.e., heterozygous hypobetalipoproteinemia) maintain normal adrenal function.(5)
In-vivo observations also suggest circulating LDL-C may contribute to adrenal steroidogenesis in humans. Following maximally stimulated cortisol output in healthy subjects and those with adrenal adenoma, plasma concentrations of apoB, but not HDL-C or HDL- and LDL-particle concentrations, were lower in samples obtained from the adrenal veins compared with the inferior vena cava.(7) In contrast to absent or near absent levels of circulating LDL-C observed in those with hypobetalipoproteinemia, individuals with familial hypercholesterolemia (FH), characterized by high circulating levels of LDL-C secondary to well defined genetic mutations (LDLR, APOB, and PSCK9) which limit adrenal uptake of LDL-derived cholesterol, exhibit normal adrenal function.

In addition, individuals achieving very low LDL-C with the use of lipid-lowering medications, including statins and PCSK9 inhibitors, also appear to have normal adrenal function. In 2008, Sezer et al. observed normal adrenal function in individuals with LDL-C levels <70 mg/ dL.(8) More recently, analysis of the DESCARTES study demonstrated very low levels of LDL-C can be attained while maintaining normal adrenal function. Of those treated with Evolocumab, 87% achieved LDL-C levels <40 mg/dL (40% <15 mg/dL) with no adverse effects involving adrenal function.(9, 10)
The second of the two primary pathways, and the most important pathway for cholesterol bioavailability in the adrenals of rodents (11), SR-B1 mediates the transfer of HDL-derived cholesterol esters to the cell membrane.(4) The adrenal glands of HSL knockout mice demonstrate significantly impaired steroid production, secondary to an inability to process and utilize cholesteryl esters selectively derived from lipoproteins, while steroid production is normal in mice lacking LDL receptors.(12) In human and mouse adrenal cells, SR-B1 expression is regulated by ACTH as demand for cholesterol availability increases.(13,14) Efflux of cellular unesterified cholesterol from cells is observed in both the basal and following ACTH simulation.(15,16)
The preferential use of LDL vs HDL as a source of cholesterol for steroidogenesis appears to be species specific with humans, pigs and cattle favoring LDL receptor-mediated endocytosis pathway; while rodent adrenals favor HDL via the SR-B1 pathway.
In 2011, Vergeer et al. identified a family with a mutation in SCARB1, encoding SR-B1.(17) Dysfunctional SR-B1 increased plasma HDL-C levels, consequently reducing adrenal intracellular cholesterol and impairing adrenal function. In contrast, studies of individuals with low HDL-C due to mutations in ABCA1 and LCAT found males had low basal but normal stimulated adrenal function (18), while females maintained normal adrenal function. (19) The authors suggested such findings highlighted 1) the importance of HDLderived cholesterol in the adrenal cell; 2) differences in adrenal function due to low cholesterol availability caused by low levels of circulating HDL vs SR-B1 dysfunction; and 3) potential differences in cholesterol metabolism or demand between males and females.
The importance of proteins in trafficking free cytosolic cholesterol to the mitochondria has been shown in mouse models, in which absent/inactive HSL results in increased adrenal accumulation of intracellular cholesterol along with impaired corticosterone production.(4,20) It appears that under physiologic conditions, the supply of cholesterol is not rate limiting, since multiple sources are available to provide ample cholesterol for steroid biosynthesis. Although de-novo intracellular synthesis and the cholesterol esters of lipid droplets are capable of providing adequate amounts of cholesterol, the current evidence suggests the human adrenals preferentially acquire cholesterol from LDL and other apolipoprotein B- and E-containing lipoproteins via LDL (B/E) receptor mediated endocytic pathways.(4)
SUMMARY
The vast majority of those with genetic disorders of lipid and lipoprotein metabolism have normal adrenal function. Because of the critical role of the adrenals, humans appear to have several redundant mechanisms for ensuring an adequate supply of cholesterol for steroid production. However, reports of adrenal insufficiency in individuals with a limited number of rare genetic causes are concerning. While many of these observations were made using older testing methods and assays, they potentially have important implications for individuals with severe LDL/HDL deficiency.
Further research is needed since it remains unclear as to whether adrenal impairment, if present in those with absent or near absent LDL/HDL, 1) is a direct consequence of lack of cholesterol or indirectly caused by secondary effects, 2) affects basal cortisol requirements, cortisol production during stress, or both, or 3) is age and/or sex specific.
While it is reassuring that individuals with untreated heterozygous FH and those who achieve very low levels of LDL-C (i.e., <15 mg/dL) on treatment both exhibit normal adrenal function, this may not be true of individuals with genetic conditions which result in absent or near absent LDL or HDL. The latter should be managed with caution, especially when experiencing stress. Further studies are needed to help guide clinical decision-making, including the potential benefit of testing adrenal function, monitoring for signs and symptoms of adrenal insufficiency and the role, if any, of steroid hormone replacement therapy.
Acknowledgements: This manuscript was conducted as part of the Pediatric Research Program (PRP), a collaboration between University of North Texas Health Science Center and Cook Children’s Healthcare System. The authors would like to acknowledge Suzanne Beckett, Dena Hanson, and Ashley Brock for their assistance in preparing and editing this manuscript.
Disclosure statement:
Dr. Hamilton has no financial disclosures to report. Mr. Jack has no financial disclosures to report. Dr. Wilson has no financial disclosures to report
References:
1. Rone MB, Fan J, Papadopoulos V. Cholesterol Transport in Steroid Biosynthesis: Role of Protein-Protein Interactions and Implications in Disease States. Biochim Biophys Acta 2009;1791(7):646-658.
2. Connelly MA, Williams DL. SR-BI and Cholesterol Uptake Into Steroidogenic Cells. Trends Endocrinol Metab 2003;14(10):467- 472.
3. Connelly MA, Kellner-Weibel G, Rothblat GH, et al. SR-BI-directed HDL-cholesteryl Ester Hydrolysis. J Lipid Res 2003;44(2):331-341.
4. Hu J, Zhang, Z, Shen WJ, et al. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr Metab 2010;7(47).
5. Illingworth DR, Orwoll ES, Connor WE. Impaired cholesterol secretion in abetalipoproteinemia J Clin Endocrinol Metab 1980;50(5):977-999.
6. Illingworth DR, Kenny TA, Orwoll ES. Adrenal function in heterozygous and homozygous hypobetalipoproteinemia. J Clin Endocrinol Metab 1982;54(1):27-33.
7. Buitenwerf E, Dullaart RPF, Muller Kobold AC, et al. Cholesterol delivery to the adrenal glands estimated by adrenal venous sampling: An in vivo model to determine the contribution of circulating lipoproteins to steroidogenesis in humans. J Clin Lipidol 2017;11(3):733-738.
8. Sezer, K., Emral, R., Corapcioglu, D, et al. Effect of very low LDL-cholesterol on cortisol synthesis. J Endocrinol Invest 2008;31(12):1075-1078.
9. Blom DJ, Hala T, Bolognese M, et al. A 52-Week PlaceboControlled Trial of Evolocumab in Hyperlipidemia. N Engl J Med 2014;370:1809-1819.
10. Blom DJ, Djedjos CS, Monsalvo ML, et al. Effects of Evolocumab on Vitamin E and Steroid Levels: Results From the 52-Week, Phase 3, Double-Blind, Randomized, Placebo-Controlled DESCARTES Study. Circ Res 2015; 117(8):731-741.
11. Rigotti A, Trigatti BL, Penman M, et al. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA 1997;94(23):12610-12615.
12. Kraemer FB. Adrenal Cholesterol Utilization. Mol Cell Endocrinol 2007;265-266:42-45.
13. Shen WJ, Azhar S, Kraemer FB. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu Rev Physiol 2018;80:95-116.
14. Shen WJ, Azhar S, Kraemer FB. ACTH Regulation of Adrenal SR-B1. Front Endocrinol 2016; 7:42.
15. Cummins CL, Volle DH, Zhang Y, et al. Liver X Receptors Regulate Adrenal Cholesterol Balance. J Clin Invest 2006;116(7):1902-1912.
16. Pagler TA, Rhode S, Neuhofer A, et al. SR-BI-mediated High Density Lipoprotein (HDL) Endocytosis Leads to HDL Resecretion Facilitating Cholesterol Efflux. J Biol Chem 2006;281(16):11193- 11204.
17. Vergeer M, Korporaal SJ, Franssen R, et al. Genetic variants of the scavenger receptor BI in humans. N Engl J Med 2011;364(2):136- 145.
18. Bochem AE, Holleboom AG, Romjin JA, et al. High density lipoprotein as a source of cholesterol for adrenal steroidogenesis: a study in individuals with low plasma HDL-C. J Lipid Rs 2013;54(6):1698-1704.
19. Bochem AE, Holleboom AG, Romjin JA, et al. Adrenal function in females with low plasma HDL-C due to mutation in ABCA1 and LCAT. PLoS One 2014; 9(5).
20. Li H, Brochu M, Wang SP, et al. Hormone-sensitive lipase deficiency in mice causes lipid storage in the adrenal cortex and impaired corticosterone response to corticotropin stimulation. Endocrinology 2002;143(9):3333-3340.





.jpg)
.png)













