Nonalcoholic fatty liver disease (NAFLD) is the main hepatic complication of obesity, diabetes and metabolic syndrome. Its prevalence has risen dramatically since 1980, when it was a perplexing and sporadically-observed disease as first reported by Ludwig and colleagues (1), to a current estimated 25% global prevalence.(2) NAFLD represents the leading cause of chronic liver disease and is the second leading indication for liver transplantation in the United States.(3) Its rise parallels the obesity epidemic and is attributed to the concomitant rise in the prevalence of type 2 diabetes and metabolic syndrome. Its strongest risk factors are similar to those of cardiovascular disease (CVD). There is growing evidence that combined postprandial hyperglycemia and hypertriglyceridemia (postprandial dysmetabolism) are likely to play a significant role toward inducing NAFLD.
More recent evidence utilizing a systems biology and metagenomics approach reveals NAFLD is likely to be a component of a more systemic disease, and the pathophysiology within the liver contributes significantly to overall progression.(4-9) Other organ systems include dysfunction of the adipose tissue, skeletal muscle, pancreas, intestine and central nervous system (Figure 1). The challenge for clinicians, payers and public health officials grappling with the condition is to identify who among those with NAFLD are at greatest necessity to treat.

Figure 1. NAFLD is a component of a multisystemic disease
Adapted from Int. J. Mol. Sci. 2019, 20, 1948; doi:10.3390/ijms20081948
DISEASE SUBTYPES, NATURAL HISTORY AND COMPLICATIONS
NAFLD is fundamentally defined as an abnormal accumulation of hepatic fat in > 5% of hepatocytes. It consists of two main subtypes: simple steatosis and nonalcoholic steatohepatitis (NASH). The two subtypes are defined by specific liver histologic findings, possess different pathophysiological underpinnings and have very different clinical outcomes.
Simple steatosis is characterized by the presence of isolated hepatic steatosis without necro-inflammatory features on biopsy. It is associated with a very low risk of progressive liver disease and liver-related mortality.
NASH is characterized by the presence of hepatic steatosis accompanied by ballooned hepatocytes, which represent inflamed, injured and degenerating hepatocytes. While simple steatosis rarely leads to progressive liver injury, NASH can undergo fibrotic progression and develop into cirrhosis among 20% of affected persons.
The pathogenesis of NASH is complex and is thought to encompass dual or multiple pathogenic hits(10) (Figure 2). The first hit, postprandial dysmetabolism, serves as a permissive factor leading to triglyceride accumulation within hepatocytes. A concomitant second insult, inflammatory and oxidative stress triggers, occurs in
approximately 30% of persons leading to a dysfunctional hepatic response to excess free fatty acids, culminating in the necro-inflammatory form of disease, NASH. The exact nature of the additional hit has not been fully elucidated; however, genetic factors, environmental (i.e. smoking, pollution and the intestinal microbiome), hormonal and dietary factors have been proposed. Advanced glycation end-products (AGE’s) and lipid peroxidation contribute significantly to its progression.
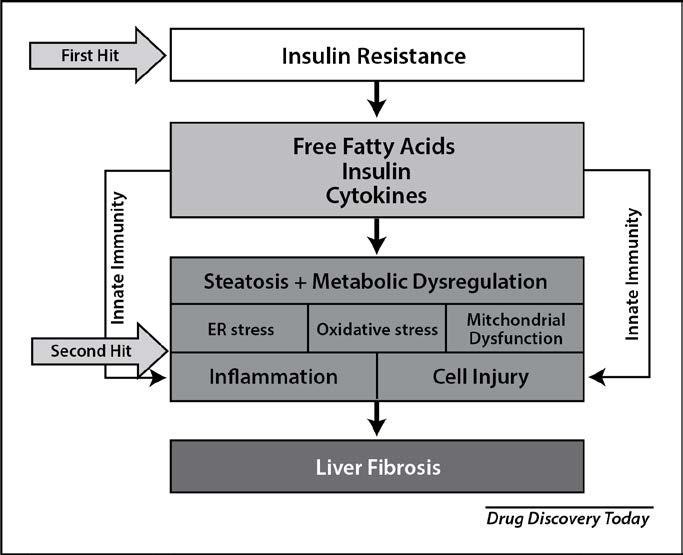
Figure 2. Transition of NAFLD to NASH – The “Two Hit” Hypothesis
Adapted from Drug Discov Today. 2019 Feb;24(2):560-566. doi: 10.1016/j.drudis.2018.09.020. Epub 2018 Oct 3.
Liver fibrosis severity is the strongest independent predictor for all-cause mortality among patients with NASH. Fibrosis is measured histologically using the METAVIR staging system that ranges from 0 (no fibrosis) and increases to stage 4 (which represents hepatic cirrhosis). Retrospective cohort studies have repeatedly demonstrated that overall mortality is increased among patients with advanced fibrosis (stage 3-4) compared with no/early fibrosis (stage 0-2) irrespective of the extent of steatosis, ballooning and inflammation.
Patients with simple steatosis are at an increased risk of cardiovascular complications compared to patients without NAFLD, particularly if there is concomitant diabetes.(11) NASH is associated with greater risk of cardiovascular complications than simple steatosis, an association that may be explained by shared risk factors.(12) The increased mortality seen among patients with advanced liver fibrosis is also associated with complications of metabolic syndrome, cardiovascular disease, chronic kidney disease and cancer.(13)
DIAGNOSIS
Making an initial diagnosis of NAFLD requires meeting two criteria: demonstration of hepatic steatosis (by imaging or liver biopsy) and exclusion of secondary causes of hepatic steatosis or alternate causes of liver disease (Table 1). Abdominal ultrasound is favored for determining the presence of hepatic steatosis. After the presence of hepatic fat is confirmed, a thorough evaluation should be done to exclude alternative causes of hepatic steatosis and chronic liver disease (if elevated liver enzymes are present) as outlined in Table 1. The most frequent secondary causes of hepatic steatosis include hepatitis C infection, excessive alcohol intake and specific medications including amiodarone, tamoxifen, methotrexate and steroids. Multiple causes might be identified but difficult to tease apart.
Table 1. Secondary causes of hepatic steatosis and liver disease - workup
Elevated liver enzymes are common in NAFLD but not a necessary diagnostic feature. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) typically range from normal to 250 IU/mL, with an AST/ALT enzyme ratio < 1. More significantly elevated liver enzymes should raise the possibility of an alternative diagnosis and an AST/ALT ratio > 1 may indicate the presence of cirrhosis. ALT and AST levels are not reliable indicators of the presence or severity of NAFLD.(14)
“ALT and AST levels are not reliable indicators of the presence or severity of NAFLD.”
RISK STRATIFICATION
Liver biopsy is the gold standard to stratify NAFLD and the only way to identify histologic ballooning to diagnose NASH. However, it is impractical to biopsy everyone with NAFLD. Fortunately, there are reliable noninvasive methods for prediction of advanced fibrosis. Noninvasive testing is used as the first line approach to identify patients at highest risk for advanced fibrosis and biopsy is offered to select patients who will be offered liver specific therapies.
The NAFLD fibrosis score (NFS) is the best validated and most widely used clinical prediction model. It is a formula consisting of age, body-mass-index, presence of hyperglycemia, AST/ALT ratio, platelet count and albumin (http://nafldscore. com/). An NFS score below -1.455 and above 0.676 identifies patients at a low risk and high risk for advanced fibrosis (F3/F4), respectively. Those in between are considered to be at intermediate risk. Nevertheless, the intermediate category in addition to high risk has been shown to increase the likelihood of liver related events and outcome of death or liver transplantation.(15) When the NFS score is used to choose candidates for liver biopsy, an intermediate or high risk scores (NFS score > -1.455) is used as the threshold for liver biopsy.
Transient elastography (TE) is a noninvasive ultrasound-based technique that uses mechanical vibrations to correlate increasing liver stiffness with worsening liver fibrosis. TE can be performed in an office setting and has reasonably good accuracy for predicting advanced fibrosis. However, its use is limited in persons with BMI ≥ 35kg/m2. Magnetic resonance elastography is more reliable than TE for estimating liver fibrosis and can be used in the setting of morbid obesity, but it may not be widely available at all centers.
TREATMENT
The contemporary management of NAFLD should consist of treating liver disease
as well as the associated metabolic comorbidities. Pharmacological treatments target primarily biopsy-proven NASH and fibrosis. Treatment considerations from the 2018 Practice Guidance from the American Association for the Study of Liver Disease (AASLD) and more recent literature reviews are provided below.
(4,16-19)
Promote Healthy Lifestyle - Weight Loss, Diet and Exercise
Weight reduction is the cornerstone of therapy. A loss of 5% of the patient’s baseline weight is associated with reduction in hepatic steatosis, 7% with improvement in NASH histology, and 10% with fibrosis regression. A combination of a hypocaloric diet (daily reduction by 500-1,000 kcal) and moderate-intensity exercise is likely to provide the best likelihood of sustaining weight loss over time. While firm recommendations regarding specific dietary patterns are lacking, the Mediterranean diet is the most suggested for patients with NAFLD. Exercise alone in adults with NAFLD may prevent or reduce hepatic steatosis, but its ability to improve other aspects of liver histology remains unknown.
Control Factors That Degrade Hepatic and Overall Health - Smoking, Alcohol and Infectious Disease
Smoking is linked to progression of fibrosis so cessation should be strongly encouraged. Alcohol exposure may exacerbate NAFLD related liver disease and increase the risk of cirrhosis. There are insufficient data to make concrete recommendations with regard to how much alcohol use is safe among individuals with NAFLD. Therefore, in clinical practice, the approach is to counsel patients regarding the risk of concomitant alcohol exposure and NAFLD and to advise alcohol avoidance. In addition, all patients with NAFLD should be vaccinated for hepatitis A and B to minimize risk of additional hepatic injury.
Manage Risk Co-Factors for CVD and CKD
Patients with NAFLD are at high risk for cardiovascular and renal morbidity and mortality. Along with lifestyle changes, aggressive glucose, blood pressure and lipid management should be considered in all patients with NAFLD.
Table 2. Management of NAFLD
Surgery - Bariatric Surgery and Liver Transplant
NAFLD is not an indication for bariatric surgery. More investigation is required to understand the full clinical utility of bariatric surgical management for management of NAFLD. However, foregut bariatric surgery can be selectively considered in otherwise eligible obese individuals with NAFLD but without advanced cirrhosis. Liver Transplant is Class 1-A indication for advanced decompensated NASH cirrhosis.
“The disease spectrum of NAFLD encompasses a wide range of patients, and it will continue to represent a growing public health burden of epidemic proportion.”
Pharmacologic Therapy
Care should be taken to avoid adverse effects of drugs that could potentially injure the liver, kidney or heart. Overall, solid data for existing drugs is lacking so care should be taken until further evidence is obtained.
Vitamin E
Vitamin E (a-tocopherol) administered at a daily dose of 800 IU/day improves liver histology in nondiabetic adults with biopsy-proven NASH and therefore may be considered for this patient population. Vitamin E is not recommended to treat NASH in diabetic patients, NAFLD without liver biopsy, NASH cirrhosis or cryptogenic cirrhosis.
Table 3. Drugs in Development to Treat NASH
Diabetes Agents
Pioglitazone, a PPAR-γ agonist, improves liver histology in patients with and without T2DM with biopsy-proven NASH. However, heart failure, fluid retention, subcutaneous fat weight gain, bone demineralization and anemia are known adverse effects. At this time, pioglitazone should be used to only treat biopsy-proven NASH. GLP-1 agonists are promising agents but cannot specifically be recommended for NASH at this time. Metformin and other antidiabetic agents are not recommended specifically to treat NAFLD or NASH but may be used if otherwise indicated.
Statins and Other Lipid Lowering Agents
Patients with NAFLD or NASH are not at higher risk for serious liver injury from statins, so they can be used to treat dyslipidemia in patients with NAFLD and NASH. However, they should be avoided with decompensated cirrhosis. Fenofibrate, a PPAR a agonist, may convey benefit but further studies are required. Ezetimibe and omega-3 fatty acids should not be used as a specific treatment of NAFLD or NASH, but they may be considered in patients with dyslipidemias. Initial studies evaluating PCKS9 Inhibitors are promising but no recommendation can be made.
Weight Loss Agents
Orlistat and other weight loss drugs are not recommended to specifically treat NASH.
Future Drugs in Development
While lifestyle intervention can reverse NASH and fibrosis, only a minority of patients can achieve sufficient weight reduction and histological improvement. A variety of drug targets are being pursued (Table 3) both as monotherapy and in combination with 2-3 other agents. At present, five drugs have entered phase 3 development for the treatment of NASH, but the selonsertib Phase 3 program has been recently discontinued.
CONCLUSION
The disease spectrum of NAFLD encompasses a wide range of patients, and it will continue to represent a growing public health burden of epidemic proportions. Prevention and lifestyle management are still the foundation
of current therapy. Therapies aimed
at controlling metabolic syndrome and reducing cardiovascular and renal risk should be utilized throughout all stages for NAFLD and NASH. Unfortunately, the current therapies have limited efficacy, although sustained weight loss can alter the natural history of disease. Although all patients with NAFLD should receive thoughtful clinical and supportive care, patients with NASH should be treated more aggressively, due to the natural course of this disease. Future pharmacologic strategies to treat NASH are underway with the first new agent being potentially released in 2020. They will target metabolic pathways that impact insulin sensitivity, fatty acid synthesis and oxidation, and various mechanisms that decrease inflammation and fibrosis. Ultimately, the horizon is bright for better and more precise treatment options.
Disclosure statement: Dr. Balakrishnan received honoraria from Intercept, Gilead, and Ebix Reviews. Dr. Neff has no financial disclosures to report.
REFERENCES
1. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434-8.
2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global Epidemiology of Non-Alcoholic Fatty Liver Disease–Meta-Analytic Assessment of Prevalence, Incidence and Outcomes. Hepatology (Baltimore, Md). 2015:n/a-n/a.
3. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188-95.
4. Black D, Brockbank S, Cruwys S, Goldenstein K, Hein P, Humphries
B. The future R&D landscape in non-alcoholic steatohepatitis (NASH). Drug Discov Today. 2019 Feb;24(2):560-566. doi: 10.1016/j.drudis.2018.09.020. Epub 2018 Oct 3.
5. Nielsen J: Systems Biology of Metabolism: A Driver for Developing Personalized and Precision Medicine. Cell Metab. 2017; 25(3): 572–9.
6. Marschisello S, Di Pino A, Scicali R, et al. Pathophysiological, molecular and therapeutic issues of nonalcoholic fatty liver disease: an overview. Int J Molec Sci. 2019;20:1948.
7. Nakamura A, Sato K, (23) Carobbio S, Pellegrinelli V, Vidal-Puig A: Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome. Adv Exp Med Biol. 2017; 960: 161–96.
8. Lovric A, Granér M, Bjornson E, et al.: Characterization of different fat depots in NAFLD using inflammation-associated proteome, lipidome and metabolome. Sci Rep. 2018; 8(1): 14200.
9. Kahn CR, Wang G, Lee, K et al. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J Clin Invest. 2019;129(10):3990-4000.
10. Patel SS, Siddiqui MS. Current and Emerging Therapies for Non-alcoholic Fatty Liver Disease. Drugs. 2019 Jan;79(1):75-84. doi: 10.1007/s40265-018-1040-1.
11. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018 Jan;67(1):328-357. doi: 10.1002/hep.29367. Epub 2017 Sep 29.
12. Oni ET, Agatston AS, Blaha MJ, Fialkow J, Cury R, Sposito A, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis. 2013;230(2):258-67.
13. Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology. 2017;65(5):1557-65.
14. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. Jama. 2015;313(22):2263-73.
15. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782-9.e4.
16. Thiagarajan P, Aithal GP. Drug Development for Nonalcoholic Fatty Liver Disease: Landscape and Challenges. J Clin Exp Hepatol. 2019 Jul-Aug;9(4):515-521. doi: 10.1016/j.jceh.2019.03.002. Epub 2019 Mar 13.
17. Vizuete J, Camero A, Malakouti M, Garapati K, Gutierrez J. Perspectives on Nonalcoholic Fatty Liver Disease: An Overview of Present and Future Therapies. J Clin Transl Hepatol. 2017 Mar 28;5(1):67-75. doi: 10.14218/JCTH.2016.00061. Epub 2017 Mar 20.
18. Sumida Y, Okanoue T, Nakajima A; Japan Study Group of NAFLD (JSG-NAFLD). Phase 3 drug pipelines in the treatment of NASH. Hepatol Res. 2019 Sep 8. doi: 10.1111/hepr.13425. [Epub ahead of print] Review. PMID: 31495973
19. Wong VW, Singal AK. Emerging medical therapies for non-alcoholic fatty liver disease and for alcoholic hepatitis. Transl Gastroenterol Hepatol. 2019 Jul 19;4:53. doi: 10.21037/tgh.2019.06.06. eCollection 2019. Review. PMID: 31463412






.jpg)
.png)












