As clinical lipidologists, we long have aspired to treat the most common cause of mortality, atherosclerotic cardiovascular disease (ASCVD). Along the way we also have been treating diabetes, when other providers may not have been managing blood glucose in addition to lipid management.1 To further complicate the clinical picture, it appears that our primary therapy – statins – has the potential to increase the risk of new onset diabetes in patients at high risk.2 The mechanisms for this are beyond the scope of this article, but it has been suggested that some statins are toxic to beta cells in the pancreas.3
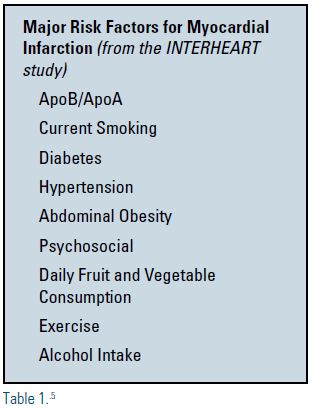
It is well known that diabetics have a higher risk of developing ASCVD and, in fact, most diabetics die from cardiovascular-related diseases.4 A review of risk factors that contribute to myocardial infarction (MI) has been observed in the Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (INTERHEART) study5, a global case control study of risk conducted in 52 countries. This trial identified nine risk factors that account for more than 90% of all risk for acute MI in the countries studied (see Table 1).5 The combination of dyslipidemia as reflected in the apolipoprotein B/apolipoprotein A (apoB/apoA) ratio and diabetes alone accounts for approximately 60% of the population’s attributable risk for acute MI. Until recently, there has been no data that demonstrate treating the glucose component of the diabetic patient could result in reductions of macrovascular events. Two new landmark trials have changed the landscape for these patients and for the clinicians who treat them.
Recent data from two large clinical trials – Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG)6 and Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (LEADER)7 – now have demonstrated that glucose control with these agents can result in reductions in ASCVD events. As a result, now is the time for the clinical lipidologist to move glucose control to the forefront, equally important to the control of dyslipidemia. These conditions should no longer be segmented and treated by different specialties. They are interrelated and both contribute to diabetic dyslipidemia, with the lipid risk greatly enhanced in the presence of elevated glucose.
In our clinics, we regularly see diabetic patients with dyslipidemia who also have elevated glucose, A1c and triglycerides, in addition to not being at low-density lipoprotein cholesterol (LDL-C) goal. These patients represent a special therapeutic challenge, because the resultant hypertriglyceridemia is associated with a higher risk of cardiovascular disease8 and the diabetic patient is most likely to have risk not captured by standard measurements of LDL-C.9
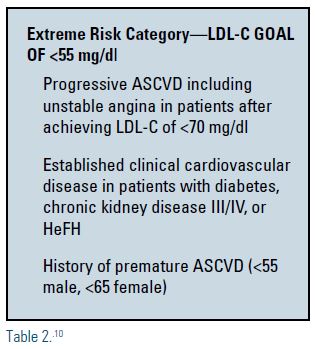
Indeed, the most recent guidelines from the American Association of Clinical Endocrinologists advocates a new LDL-C goal of <55 mg/dL in patients who are at highest risk, including diabetics with established clinical ASCVD, as well as those with chronic kidney disease, heterozygous familial hypercholesterolemia, and premature ASCVD (see Table 2).10 I am happy to embrace this new extreme-risk category and I believe it underscores the concept that lower is better.
The Empaglifozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes (EMPA-REG) trial included more than 7,000 subjects with type 2 diabetes at high cardiovascular risk. The subjects were treated in this placebo-controlled outcomes trial with empaglifozin added to standard care for a mean of 3.1 years. This trial met its primary composite outcomes of death from cardiovascular causes, nonfatal MI or nonfatal stroke. There was a 38% relative risk reduction (2.2% absolute risk reduction) in death from cardiovascular causes. The number needed to treat to prevent one event over a three-year period is 38 in the 25 mg empaglifozin group.
It is interesting to note that the authors of this trial can only speculate about the mechanisms of the achieved risk reductions, citing changes in arterial stiffness and cardiac function, cardiorenal effects, reductions in albuminuria and uric acid, and positive effects on hyperglycemia, weight, visceral adiposity and blood pressure.
As far as lipid parameters, there were small increases in both LDL-C and high-density lipoprotein cholesterol (HDL-C) in this trial. It would be clinically useful if these parameters could be included in future trials to more precisely examine how glucose control affects advanced lipoprotein measurements. The reduction in triglycerides that accompanies the improvement in blood glucose has a salutary effect on HDL-C (increase), and LDL-P (decrease in number and increase in size).
The Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes (LEADER) trial was conducted on 9,340 subjects with type 2 diabetes and high cardiovascular risk defined by age greater than 50 with ASCVD, chronic kidney disease stage 3 or greater, or New York Heart Association (NYHA) class II-III chronic heart failure. Liraglutide, a glucagon-like peptide analogue (GLP-1 agonist), was compared to placebo as add-on therapy to standard care in this population for a mean study duration of 3.8 years. The primary composite outcome in the time-to-event analysis was the first occurrence of death from cardiovascular causes, nonfatal MI, or nonfatal stroke. The study drug resulted in a 13% reduction in the primary composite outcome versus placebo. There was a 22% reduction in death from cardiovascular causes and a 14% reduction in overall MI. The number needed to treat to prevent one event in three years was 66 for the primary outcome and 98 for death from any cause.
The authors of this trial propose that, unlike that seen in EMPA-REG, the benefit derived here likely is attributable to the modified progression of ASCVD. Surprisingly in this trial of high risk diabetics, only 72% of the study population was on a statin at study entry.
In summary, we now have evidence that we can impact ASCVD by lowering glucose with an SGLT-2 inhibitor and a GLP-1 agonist. As we begin to understand the complex relationships of the major risk factors for heart disease, we will be better able to formulate therapeutic regimens that address the fundamental metabolic processes involved in patients with diabetes.
Moving forward, we need clinical trials designed to study the actual pathology involved in this high-risk group. It would be of great clinical relevance to study real-world patients who suffer from elevated glucose, elevated triglycerides and elevated apoB using pharmacologic interventions that address each defect in combination. It may be more clinically useful to determine that treating the diabetic dyslipidemic as comprehensively as possible might yield better results than looking at one intervention at a time. It is certainly possible that the combination of available therapies might yield even more risk reduction than the sum of each agent individually.
The future holds much promise for the clinical lipidologist, with many new interventions in the pipeline that should only enhance our abilities to further protect this patient population from ASCVD. I look forward to data on apolipoprotein C-III (apoC3) inhibition efforts to increase lipoprotein lipase and glucokinase activators. Until we have more data and more in our toolbox, I believe we best can serve our patients by concurrently controlling every risk factor we can identify.
Disclosure statement: Dr. Lillo was on a speakers bureau for Amgen, Sanofi, Kowa and Amarin. He also participated in research trials for Amgen, Merck, Astra Zenecca, Glaxo, Braintree, Allergan, Kowa and Novartis.
References can be found here.






.jpg)
.png)