In the late 1980s and in the 1990s, the emergence of the powerful statin drugs transformed treatment of dyslipidemia. Since then they have become the standard of care for managing this condition. Not since that era has there been as much energy, excitement, and promise to further advance our efforts to treat dyslipidemia as that afforded by the development of pro-protein convertase subtilisinlike kexin type 9 (PCSK9) monoclonal antibody therapy. The most recent Phase III trial results are promising as significant advancement.
PCSK9 is a 692 amino acid mature protein, which consists of three domains: prodomain, catalytic, and its C-terminal end. It is primarily expressed as a secreted protease in the liver, intestines, and kidneys. It exhibits rapid turnover in plasma (<10 minutes), and its removal is facilitated primarily by the low-density lipoprotein (LDL) receptor. PCSK9 is a key regulator of LDL receptor expression and degradation.
LDL receptors usually recycle back to the cell surface after they deliver their load of cholesterol intracellularly. However, when secreted, PCSK9 forms a complex with the epidermal growth factor-like domain A (EGF-A) of the LDL receptor extracellular domain; and thus the PCSK9/LDL receptor complex undergoes endocytosis and subsequent degradation.1 The resulting presence of fewer LDL receptors to process LDL results in a buildup of plasma LDL. Generally, since increased PSCK9 leads to increased degradation of LDL receptors, inhibition of PCSK9 should lead to a significant decrease in lowdensity lipoprotein cholesterol (LDL-C). Importantly, use of statins and other drugs that lower LDL-C lead to an increase in PSCK9 secretion, an effect that likely decreases the ability of statins to lower LDL.
Interestingly, a number of genetic abnormalities have been noted to affect PCSK9 activity. Consistent with this hypothesis that PCSK9 is a key regulator of the LDL receptor, gain-of-function missense mutations in the PCSK9 gene have been noted to be associated with genetic hypercholesterolemia consistent with the familial hypercholesterolemia (FH) phenotype. In contrast, loss-offunction nonsense mutations have been associated with very low LDL levels and a major reduction in coronary heart disease (CHD) incidence.2,3
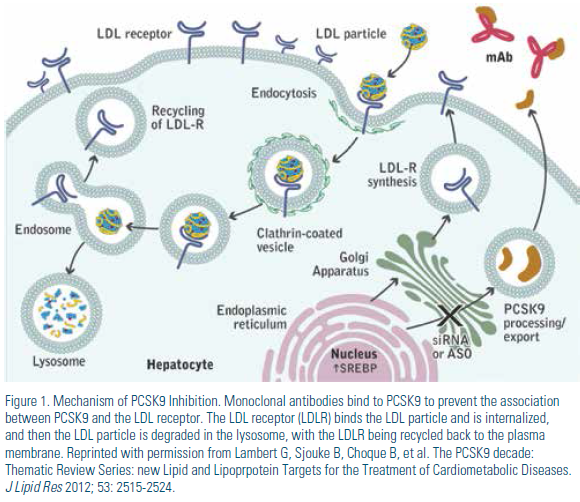
Potential therapeutic targets for inhibiting the PCSK9 pathway include 1) reducing PCSK9 protein production, 2) reducing PCSK9 mRNA expression, 3) inhibiting PCSK9 binding to the LDL receptor, and 4) inhibiting PCSK9 mediated degradation of the LDL receptor.4 Many of these strategies are currently being explored with investigational therapies. (Table 1) Of these, using monoclonal antibodies to bind PCSK9 is the strategy that has been most rigorously evaluated to date. When monoclonal antibodies bind to PCSK9, they prevent formation of the PCSK9/LDL receptor complex, leading to a reduction in the degradation of the LDL receptor, thus retaining the ability of LDL receptors to continue to internalize and degrade LDL. (Figure 1)
Lessons Learned from Phase I and II Trials of PCSK9 Monoclonal Antibody Therapy
Early human trials of PCSK9 inhibition with specifically designed monoclonal antibodies provided information regarding efficacy, safety, and tolerability of this strategy. The concepts that were tested included the duration of treatment effect, information on dosing, and intensity of LDL-C reduction with and without the co-administration of statin. For example, a series of randomized, placebo-controlled multiple-dose trials of alirocumab (REGN727) were performed in adults with heterozygous familial hypercholesterolemia (FH) who were receiving atorvastatin (n=21) and those with nonfamilial hypercholesterolemia who were receiving treatment with atorvastatin (n=30) or a modified diet alone (n=10).5 Among subjects receiving the PCSK9 inhibitor alirocumab, there were no discontinuations because of adverse events. Doses of 50 mg, 100 mg, and 150 mg reduced LDL-C from baseline by 39.2, 53.7, and 61.0 percent, respectively, as compared with placebo (P<0.001 for all comparisons). Recently, data were presented on a longterm (up to 64 weeks) extension study of alirocumab in patients with heterozygous FH; in this study LDL-C was reduced by 57 to 66 percent on top of a background of statin and other therapy.6 These and other studies with alirocumab have demonstrated consistent efficacy, safety, and tolerability.7,8
Results have been similar with other PCSK9 inhibitors in development. In Phase II trials, evolocumab (AMG 145) demonstrated up to approximately 60-percent reduction of LDL-C on top of stable statin therapy in persons with heterozygous FH,9 20 to 25 percent LDL-C reduction in those with homozygous FH,10 and 50 percent or greater LDL-C reductions in statin-intolerant patients with or without ezetimibe therapy.11 In addition, the Open Label Study of Long-Term Evaluation Against LDL-C (OSLER) extension trial of 1,104 patients demonstrated overall 52 percent reductions in LDL-C from baseline over and above any background therapy, as well as sustained improvements in lipoprotein(a) and apolipoprotein B with a similar rate of adverse events to placebo.12
There also have been important reports from Phase II studies involving bococizumab (RN316) in statin-treated patients. Here there was demonstrated similar efficacy (reductions in LDL-C of at least 50 percent), safety and tolerability.13,14 Other PCSK9 monoclonal antibody therapies, including RG7652 and LY3015014, are currently in Phase II studies.
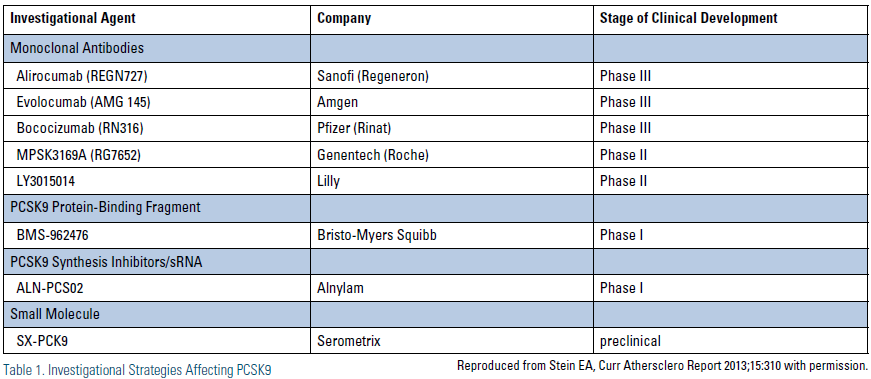
Finally, in addition to monoclonal antibodies, there are a number of other potential strategies to inhibit PCSK9 through other modalities that are in early phase clinical trials. For example, ALN-PCS02 in an investigational antisense RNA interference therapeutic that is being studied in Phase 1 studies. Other investigational products under development are listed in Table 1.
Results from Phase III Clinical Trials
Results of Phase III studies with PSCK9 inhibitors have been highly anticipated. Luckily, in the past year we have seen a number of important Phase III trial results either presented or published. The details of the Phase III results available to date with monoclonal antibodies to PCSK9 are outlined in detail below and summarized in. (Table 2)
The global Phase III program for investigation of alirocumab is known as the ODYSSEY program. Overall, the ODYSSEY program is expected to enroll more than 23,000 subjects in at least 12 planned clinical trials. Of these trials, the first to report was the ODYSSEY-MONO study, in which 103 subjects with LDL-C 100-190 mg/dl and 10-year fatal CVD risk of <5% were randomized to either alirocumab 75-150 mg dosed every two weeks or to ezetimibe 10 mg daily. Use of alirocumab led to LDL-C reductions at 24 weeks of 47.2 percent vs. 15.6 percent in those on ezetimibe.15 Importantly, many patients were able to achieve a reduction in LDL-C to below 70 mg/dl on the lower dose of 75 mg and did not require uptitration to the 150 mg dose. In general, safety and tolerability of alirocumab was comparable to, or better than, ezetimibe. Another recent one-year open-label study showed further reductions in LDL-C of 57 to 66 percent with alirocumab 150 mg Q2w beyond a statin and ezetimibe in patients with heterozygous FH.16 Finally, in a placebo-controlled trial on top of atorvastatin therapy, alirocumab 150 mg Q2w was shown to significantly reduce mean LDL particle number (LDL-P) by 63 percent as well as increase HDL particle number (HDL-P) by 11 percent.17
The Phase III clinical trial program with evolocumab is known as the Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 in Different Populations (PROFICIO). Recently, a number of Phase III clinical trials with evolocumab have been reported in a wide variety of patient populations. In the LAPLACE-2, study, 1,896 patients with primary hypercholesterolemia were initially randomized to one of five background statin treatments and then further randomized to receive different doses of evolocumab, ezetimibe, or placebo in addition to statin therapy.18 Overall, use of evolocumab demonstrated reductions in LDL-C of 55 to 76 percent compared to placebo and 38 to 47 percent compared with ezetimibe.
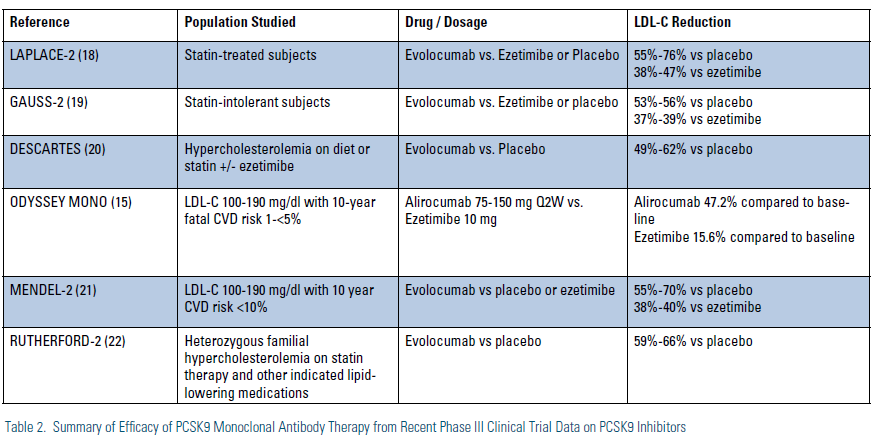
The GAUSS-2 trial randomized 307 subjects with hypercholesterolemia who were unable to tolerate effective doses of statins on at least two occasions in the past to varying doses of evolocumab, ezetime, or placebo.19 In this study, the mean baseline LDL-C of 193 mg/dl was decreased by 53 to 56 percent from baseline with evolocumab dosed once or twice a month, which was a 37 to 39 percent greater reduction than seen in those treated with ezetimibe. Of the evolucumab-treated patients, 80 to 90 percent of moderate-risk patients and 75 percent of high-risk patients achieved an LDL target of <100 mg/dl compared to only 10 percent of ezetimibe patients.
The Durable Effect of PCSK9 Antibody Compared with Placebo Study (DESCARTES)20 reported on 901 patients given evolocumab 420 mg or placebo every four weeks on top of dietary therapy, atorvastatin 10 mg plus dietary changes, atorvastatin 80 mg or atorvastatin 80 mg plus ezetimibe. Overall, use of evolocumab resulted in LDL-C reductions of 49 to 62 percent reduction across the four groups. In the Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid Levels-2 (MENDEL-2) trial,21 614 subjects with LDL-C 100-190 but relatively low cardiovascular risk not on statin therapy were randomized to one of six treatment groups to compare two dosing regimens (140 mg subcutaneously every two weeks or 420 mg subcutaneously every four weeks) with placebo or ezetimibe. In this study, use of evolocumab reduced LDL-C by 55 to 70 percent versus placebo and 38 to 40 percent more than with ezetimibe.
Finally, the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study-2 (RUTHERFORD-2)22 randomized 329 patients with heterozygous FH who were on background therapy that usually included statins to evolocumab or placebo, and demonstrated a 59 to 66 percent reduction in LDL-C over and above statin and/or other lipid-lowering therapy. In all five reported evolocumab Phase III studies that have reported to date, safety and tolerability were generally comparable to placebo or ezetimibe comparators.
Outcomes Studies
Important for determining the future role of these agents are clinical outcomes studies designed to examine whether LDL-C reduction from PCSK9 inhibitor therapy, alone or in addition to background statin use, results in further reduction of clinical cardiovascular events. A number of clinical outcome studies with PCSK9 inhibitors are planned and, in many cases, are already enrolling patients. For instance, the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) study is a secondary-outcomes study planning to enroll 22,500 subjects that will test the hypothesis of whether evolocumab, when used in addition to other dyslipidemia treatments — including statins — will reduce the risk of cardiovascular death, myocardial infarction, hospitalization for unstable angina or coronary revascularization in subjects who are known to have clinically evident cardiovascular disease.
The ODYSSEY OUTCOMES study also is a secondary-prevention trial that is planning to enroll 18,000 patients and will compare the effect of alirocumab and placebo on the occurrence of cardiovascular events in patients who have had acute coronary syndrome from four to 52 weeks prior to enrollment and are already on other appropriate lipid-lowering medications, including statins. Lastly, the SPIRE 1 and SPIRE 2 trials will examine the effect of bococizumab as compared to placebo in more than 22,000 patients at high risk for cardiovascular events who are already on appropriate lipid-lowering agents, including statins.
Clinical Perspective
In keeping with other contemporary guidelines, the recently released 2013 American College of Cardiology/ American Heart Association Guideline on Cholesterol Management confirms that statins will remain first-line therapy for the treatment of dyslipidemia in patients at elevated cardiovascular risk.23 Extrapolations from these guidelines suggest that up to 56 million U.S. adults may be eligible for statin therapy.24 What is clear, however, is that up to 20% of such statin-eligible individuals may either be intolerant to the agents and doses of statin therapy needed to achieve the desired therapeutic response in LDL-C reduction recommended by the new guideline (30–50% on a moderate-intensity statin or at least 50% from a high-intensity statin). Moreover, persons with homozygous or heterozygous FH are well-known to have a reduced response to statin therapy because of their lack of functional LDL receptors and often are far from optimal LDL-C (or non-HDL-C) despite the use of highintensity statin therapy. The evidence from the latest clinical trials suggests these groups — those who are statin intolerant and those with familial hypercholesterolemia — as ideal initial candidates for consideration of PCSK9 inhibitor therapy, either alone or in addition to the maximally tolerated dose of statin therapy. The excellent safety and tolerability profile thus far demonstrated has further suggested the promise of these therapies for these groups of patients and, in particular, for the statinintolerant individual for whom no suitable therapeutic option exists at present. However, even with a patient labeled as “statin intolerant,” multiple attempts should be made to get that patient on statin therapy, even a low dose, before consideration of other therapies, including PCSK9 inhibitors when and if they become available.
No other attractive options exist, so we suspect and encourage that PCSK9 inhibitors, when and if approved, will quickly be used in statin-intolerant or FH patients, or for those who do not reach recommended levels of therapeutic response on the basis of statins alone. Whether these agents have a role in secondary prevention for patients at high cardiovascular risk already on high-potency statins remains unclear until we have the results of cardiovascular outcomes studies currently under way. Unfortunately, these critical studies are unlikely to report results prior to 2017.
One intriguing finding of our clinical trials to date with PCSK9 inhibitors has been the effects on lipoprotein(a). Based on our current understanding of lipoprotein(a) physiology and PCSK9 functionality, use of PCSK9 inhibitors would not be expected to have a considerable effect on lipoprotein(a). However, in pooled analysis of Phase II studies with evolocumab, lipoprotein(a) levels have been reduced 25 to 30 percent as compared to placebo.25 Similar findings have been demonstrated in Phase III studies with evolucumab and with the other PCSK9 inhibitors in development. Whether these agents will have a role in treatment of patients with high lipoprotein(a) is an important clinical question to be answered in future clinical trials.
Summary
In summary, investigational PCSK9 inhibitors have thus far demonstrated tremendous efficacy in lowering LDL-C with an excellent safety and tolerability profile, either as monotherapy or in addition to statin therapy. Based on these results and the proof of concept supplied by naturally occurring genetic mutations, these products appear to be uniquely positioned to become an important part of our armamentarium in the treatment of dyslipidemia. Importantly, in just a few years, the results of clinical outcomes studies should be available to help us answer the important question of whether there is further incremental clinical event reduction from achieved LDL-C levels beyond that obtained from high-intensity statin use. Making further headway into addressing residual risk that has, thus far, not been achievable from other add-on lipid modifying agents would be truly revolutionary.
Disclosure statement: Dr. Bloch or his spouse have received honorarium, consulting fees, or research support from AstraZeneca, Amgen, Takeda Pharmaceuticals, Dailchi-Sankyo, Arbor Pharmaceuticals, Liposcience, Aegerion, Esai, Pfizer/ BMS, EMD/Serano, Novartis, Genzyme, Biogen, and Janssen. Dr. Wong has received salary through his institution from Amgen, Bristol-Myers Squibb, Gilead, and Regeneron. Dr. Wong has received consulting fees from Aviir and Amgen.
References are listed on page 35 of the PDF.





.jpg)
.png)












