The origins of the field of antisense technology date back to 1978, when Zamecnik — who 22 years earlier made the landmark discovery of transfer ribonucleic acid (tRNA) — and Stephenson first reported the ability to inhibit transcription, translation, and replication of Rous sarcoma virus in chicken fibroblasts with the use of small complimentary oligonucleotides.1 Zamecnik subsequently used antisense oligonucleotides to block replication of influenza, human immunodeficiency virus (HIV), Mycobacterium tuberculosis, plasmodium species, and others.2 In 1987 he was the first to describe the existence of microRNAs (miRNA), which are endogenous regulators of gene transcription and translation.2
In 1992, only 14 years after the initial report of gene silencing by Zamecnik and Stephenson, the journal Science designated the burgeoning field of antisense technology and the first experimental use of antisense compounds in humans as one of the top 10 most promising advances in scientific research that year.3,4 As an additional sign of the importance of this field, the 2006 Nobel Prize in Medicine was awarded to Andrew Fire and Craig Mello for the discovery of RNA interference, the process of gene silencing by double-stranded RNA.5,6
In principle, the messenger RNA (mRNA) of any gene can be blocked with antisense technology, but there are numerous technical hurdles that must be surmounted to produce an efficacious and welltolerated pharmaceutical agent that can reach the target organ and successfully bind and block the target mRNA. Although the realm of antisense development dates back several decades, the first antisense drug in clinical use, fomivirsen (Vitravene), was not FDA-approved for treatment of cytomegalovirus (CMV) retinitis in immunocompromised patients until 1998. The second antisense drug in clinical use, mipomersen (Kynamro), was approved only last year — in January 2013 — for treatment of homozygous familial hypercholesterolemia (FH). The use of fomivirsen subsequently declined after the advent of highly active antiretroviral therapy (HAART) for treatment of (HIV) infection, which virtually eliminated the occurrence of CMV retinitis. The use of mipomersen continues.
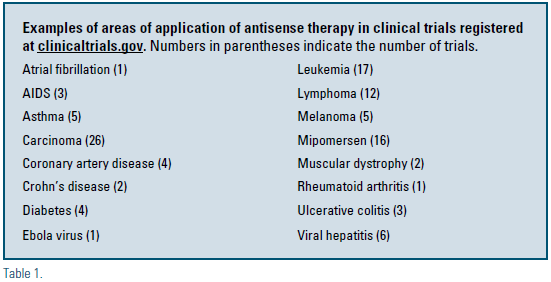
Many new antisense drugs are in various stages of clinical development and are anticipated to greatly expand the breadth of therapeutic interventions that are available for prevention and treatment of disease. ISIS Pharmaceuticals, founded in 1989 by Stanley Crooke, has been a leader in the field, but many other companies have been active in antisense drug development (e.g. Sarepta Therapeutics, Antisense Pharma, Antisense Therapeutics, Gene Signal, Prosensa, Hybridon, Alnylam, and others). There are currently 124 clinical trials related to antisense therapy that are registered at clinicaltrials.gov and are either active, completed, or terminated.7 Examples of areas of investigation are shown in Table 1. Studies also are in progress testing the efficacy and safety of antisense oligonucleotides targeting apolipoprotein CIII (apo CIII) for treatment of hypertriglyceridemia, apolipoprotein(a) [apo(a)] for treatment of elevated lipoprotein(a), C-reactive protein (CRP) for modulation of inflammation and development of atherosclerosis, and other targets related to atherosclerosis and diabetes. This field represents an exciting and promising new era of targeted drug development and disease management.

The basic principle of antisense technology is the suppression of gene transcription or mRNA translation with an interfering short sequence of bases that is complementary to the gene of interest. For readers who are unfamiliar with the terminology in this field, some of the terms are defined in Table 2. In most cases, the mRNA is targeted with an oligonucleotide that blocks translation and typically targets the mRNA for degradation. Antisense oligonucleotides (ASOs), such as mipomersen and other ASOs in development, are designed to bind a complimentary sequence of mRNA and block translation, but also target the mRNA for degradation typically by RNAse H, which frees the ASO molecule to repeatedly bind and degrade multiple mRNA molecules. Double-stranded small interfering RNA (siRNA) sequences block translation by binding of a single antisense strand of the siRNA to the complimentary strand of mRNA of interest, but also target the mRNA for degradation through the RNA-induced silencing-complex (RISC) pathway.
Mipomersen is a 20-base pair secondgeneration ASO that is complementary to mRNA for apoprotein B-100, the primary structural protein in very-lowdensity lipoprotein (VLDL), low-density lipoprotein (LDL), and lipoprotein(a) particles.8 Binding of mipomersen to the complimentary strand of mRNA targets the mRNA for degradation by RNAse H, which depresses production of apoprotein B-100 and decreases the plasma concentration of LDL cholesterol by 25 to 37 percent, with similar decreases in VLDL cholesterol, apoprotein B-100, and lipoprotein(a). Although mipomersen is FDA-approved at a dose of 200 mg subcutaneously weekly only for treatment of homozygous FH, the results of several six-month blinded placebo-controlled clinical trials have demonstrated efficacy in heterozygous familial hypercholesterolemia and other subjects with refractory hypercholesterolemia. The results of longterm open-label studies have demonstrated sustained efficacy and stability of sideeffects over two to four years.9,10
The future of antisense technology remains bright and has the potential to produce major breakthroughs in our ability to manage many human diseases, some of which are currently untreatable or difficult to manage with existing interventions. Expectations are high for the completion of clinical trials that will lead to clinical availability of important new antisense medications in coming years.
Disclosure statement: Dr. Duell has received honorarium from Merck, Aegerion, Genzyme, and Novartis, and has received research grants from Bristol-Myers Squibb and Genzyme.
References are listed on page 36 of the PDF.






.jpg)
.png)











