This edition of the LipidSpin is devoted to guidelines vs. guidance. We all strive to practice evidence-based medicine (EBM) and our understanding of science has moved forward at a quick pace. Multiple journals come out every week with new articles that advance medicine’s knowledge base. Large, randomized controlled trials (RCTs) get headlines when a positive or negative result is found. However, in the everyday practice of seeing patients and making treatment decisions, how applicable can a large RCT be to your particular patient population? Also, what method do you use to interpret positive and negative results within explanatory (efficacy) vs. pragmatic (effectiveness) trials (Table 1)?
 Schwartz and Leoouch first described the explanatory and pragmatic trial approaches in 1967.1 By definition, an explanatory (efficacy) trial is a traditional RCT designed to evaluate the effects of treatments and is considered the gold standard. A pragmatic (effectiveness) trial is designed to test a treatment or intervention in everyday clinical settings to allow for generalizability and maximum application. An explanatory trial has a main purpose of answering the question, “Does an intervention work under ideal conditions?”2 The main purpose of a pragmatic trial is to answer the question, “Can an intervention work under usual or real-world conditions?”2
Schwartz and Leoouch first described the explanatory and pragmatic trial approaches in 1967.1 By definition, an explanatory (efficacy) trial is a traditional RCT designed to evaluate the effects of treatments and is considered the gold standard. A pragmatic (effectiveness) trial is designed to test a treatment or intervention in everyday clinical settings to allow for generalizability and maximum application. An explanatory trial has a main purpose of answering the question, “Does an intervention work under ideal conditions?”2 The main purpose of a pragmatic trial is to answer the question, “Can an intervention work under usual or real-world conditions?”2
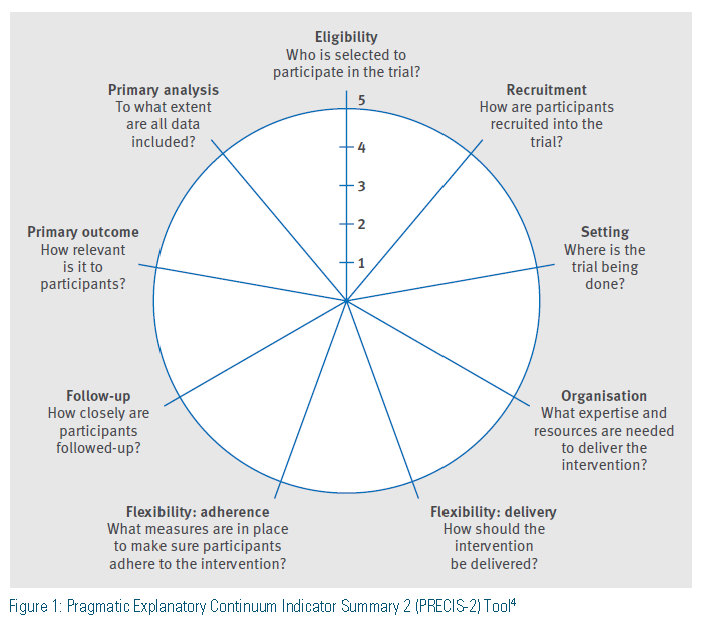
So how can a clinician compare the differences between an explanatory and a pragmatic trial? The Pragmatic Explanatory Continuum Indicator Summary (PRECIS) was developed and validated with 10 domains of focus in evaluating trial design and application and has subsequently been refined to focus on nine domains in the PRECIS-2 Tool.2-4 These key domains (Figure 1) allow for comparison between explanatory and pragmatic trials. For trials to be clinically meaningful, results must be relevant to specific patient populations in specific settings.4 Trials have many factors that determine internal and external validity, and these vary between explanatory and pragmatic trials. Some of these factors include the condition under investigation, drug regimens, compliance, patient characteristics, costs, co-morbidities, and concomitant treatment regimens.
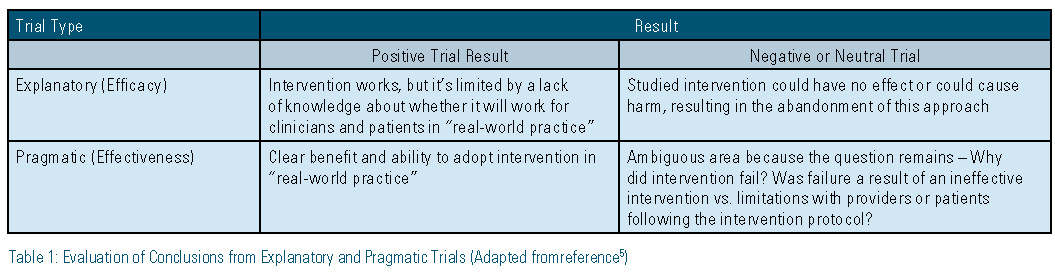
What conclusions can be drawn from explanatory versus pragmatic trials? There are advantages and disadvantages to the application of each trial type on an individual-patient level. The overall strength of explanatory trials is that a “negative” result can directly inform practitioners, because an intervention/ treatment that has been shown to not work under ideal conditions is unlikely to work under usual conditions.5 The main weakness of an explanatory trial is that a “positive” result does not always directly translate to practical use (limited external validity) because the study population may be too narrow or may include optimal circumstances that may not apply to the general population.5 Pragmatic trials essentially have the opposite strengths and weaknesses. When a “negative” result is seen in a pragmatic trial, it can be unclear as to whether the treatment/ intervention is not effective in specific patient populations because it often is studied in the general population under usual conditions. On the other hand a “positive” result can help inform individual patient- and population-based decisions because the study was performed under usual conditions, resulting in increased external validity. Table 1 provides more details on how to evaluate “positive” and “negative” outcomes within explanatory and pragmatic trials.5
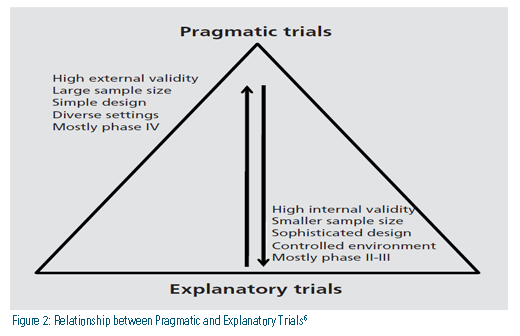
How else can the relationship between pragmatic trials and explanatory trials be explained? Figure 2 describes key differences as they relate to five factors: validity, size, design, focus population, and study type.6 Trials often are not purely explanatory or pragmatic, so what factors might suggest trial results are translatable and applicable in a primary care or specialty setting? These factors could revolve around the study design itself. For example:
 Comparison groups do not represent current standards of care
Comparison groups do not represent current standards of care- Trial protocols force uncommon clinical scenarios
- Outcomes include less meaningful end points
In addition, environment of the healthcare delivery system itself could contribute in a negative manner with the following considerations:
- Limited availability of providers or resources
- Inadequate levels of reimbursement
Thirdly, therapeutic implementation issues could be part of the problem with effectiveness trials. These might include:
- Complex and multi-faceted therapies that are challenging to implement
- The way in which the procedural experience of providers influences outcomes
Finally, patient selection issues can play a factor in pragmatic trials. These include:
- Biases in the patients who are eligible for a therapy
- Biases in patients who ultimately are selected for (or agree to) a therapy7-8
In conclusion, when reviewing a clinical trial, keep in mind the factors listed above to help you decide if the results are applicable in your particular practice setting.
Disclosure statement: Dr. Uusinarkaus has no disclosures to report. Dr. Marrs has no disclosures to report.
References are listed on page 35.
Article By:
Colorado Springs Health Partners
Adjunct Associate Professor of Family Medicine and Community Preceptor
University of Colorado, Department of Family Medicine
Colorado Springs, CO
Diplomate, American Board of Clinical Lipidology





.jpg)
.png)












