Clinical question: In statin-treated patients who also require strong CYP3A4 inducers, does statin therapy need to be preemptively modified to account for decreased systemic exposure secondary to increased metabolism?
Recent guidelines endorse the use of appropriate-intensity statin therapy to match an individual’s risk for atherosclerotic cardiovascular disease (ASCVD).1,2 The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the treatment of blood cholesterol to reduce ASCVD risk stresses the importance of considering drug-drug interactions that may influence statin safety, but no detail is provided on how to manage these interactions. The 2014 National Lipid Association Statin Safety Task Force guidelines include a detailed review of statin pharmacokinetics (PK).3 These guidelines review drug interactions that increase the risk of statin-related adverse effects, especially myopathy, which is mediated by increased systemic exposure as measured by area under the curve (AUC). On the other hand, little guidance exists to direct clinicians on how to address statin drug- drug interactions with CYP3A4 inducers that result in decreased systemic exposure.
Phase I oxidation reactions carried out by CYP3A4 are responsible for metabolizing more than 50 percent of all manufactured drugs.4 Predicting adverse events from potential CYP3A4 interactions is difficult, in part because a 10-fold difference in activity and a 40-fold difference in enzyme expression may be seen among individuals.5 PK studies have demonstrated that when CYP3A4-dependant statins (lovastatin, simvastatin and atorvastatin) are combined with strong CYP3A4 inducers (e.g. phenytoin, carbamazepine, rifampin), statin AUC is decreased by 53 percent to 82 percent (i.e. < two-fold).6,7 What effect this has on lowering low- density lipoprotein cholesterol (LDL-C) is not entirely clear.
While several case reports have suggested an association between concurrent CYP3A4 inducers and inadequate LDL-C response,8,9 it also has been demonstrated that a 60 percent reduction in the AUC of certain statins does not result in any statistically significant reduction in LDL-C lowering.10,11 Tertiary drug information resources suggest that clinicians either consider changing to a non-CYP3A4-dependant statin or monitoring for LDL-C-lowering response.5,12 It is important to recognize that a change in statin therapy may have unintended consequences.
For example, a patient being initiated on phenytoin who is preemptively switched from atorvastatin to rosuvastatin may incur substantial additional costs. Alternatively, if an ASCVD patient were to be switched to pravastatin, the patient would no longer be receiving the high-intensity statin, as recommended, and may not achieve the LDL-C goal.
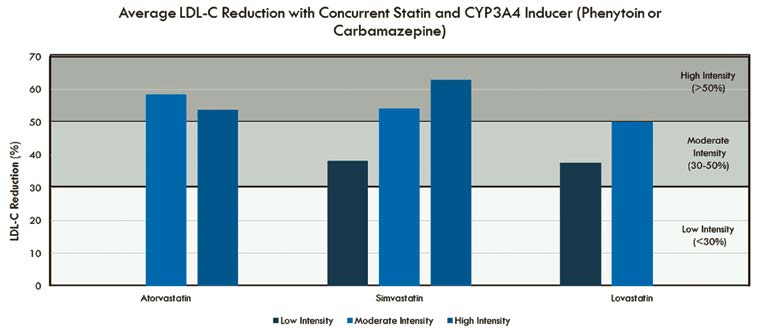
The Clinical Pharmacy Cardiac Risk Service (CPCRS) at Kaiser Permanente Colorado currently manages more than 16,000 patients with ASCVD.13 Based on a cursory review of 84 patients on a CYP3A4- dependent statin plus either phenytoin and/or carbamazepine, we determined that the vast majority of patients (94 percent) had attained at least the anticipated percentage of LDL-C reduction based on their individual statin intensity (Figure 1). There were instances in which patients appeared to have a less than or greater than anticipated LDL-C reduction based on statin intensity, which we presume was likely because of variables such as adherence to medication and/or lifestyle.
Statin drug interactions involving CYP3A4 inducers generally are less of a concern than interactions with inhibitors. Based on our collective experience at CPCRS, we feel it is unnecessary to preemptively modify a patient’s statin or dose to accommodate a concurrent CYP3A4- inducer interaction. Overall, patients in our practice seemed to respond as expected to statin therapy when co- administered with CYP3A4 inducers. These observations warrant further investigation in a prospectively designed study to confirm their validity. A final consideration is that a change in therapy may have cost implications and or lead to patients being placed on a lower-than-recommended intensity of statin. Given that there is always the potential for variation in patient response to drug therapy, monitoring for statin efficacy via fasting lipid panel — to ensure adequate LDL-C response to statin therapy — is recommended.
Disclosure statement. Dr. Lamprecht has no disclosures to report. Dr. Todd has no disclosures to report.
References are listed on page 35.
Article By:
Clinical Pharmacy Cardiac Risk Service
Kaiser Permanente of Colorado
Clinical Assistant Professor
University of Colorado Skaggs School of Pharmacy and Pharmaceutical Sciences
Aurora, CO
Diplomate, Accreditation Council of Clinical Lipidology






.jpg)
.png)












