Case:
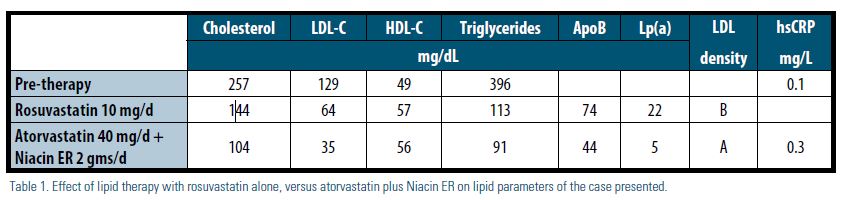
We report on a 77-year-old Caucasian male with coronary disease and hereditary dyslipidemia requiring intervention. He was treated with rosuvastatin 10 mg daily. This resulted in remarkable improvement of his low-density lipoprotein (LDL-C) to < 70 mg/dL and triglycerides (TG) to < 150 mg/dL, however, he continued to have elevated lipoprotein(a) [Lp(a)] with small, dense LDL — pattern B. A few years later, he required a carotid endarterectomy (CEA) for progressive symptomatic carotid artery disease; he also had angiography evidence that his coronary disease was progressing, though not yet requiring intervention. He was referred for specialized lipid therapy, during which he was switched to atorvastatin 40 mg daily and was slowly titrated on extended- release niacin up to 2,000 mg daily. This resulted in a remarkable decrease in his Lp(a) and normalization of his LDL density pattern to large, buoyant LDL — pattern A, as illustrated in Table 1. He had no recurrent events in the few years since this change. This uncommon case of dyslipidemia demonstrates the importance of addressing residual risk, which would require tailored individual therapy — often with combination therapy — despite achieving a seemingly well-controlled, guideline-recommended lipid profile with statin therapy alone (Table 1).
An extensive body of evidence exists supporting the safety1 and superiority of therapy with 3-hydroxy-3-methyl-glutaryl- CoA reductase (HMG-CoA reductase) inhibitors (statins) in the primary2 and secondary3 prevention of cardiovascular events. Recent ACC/AHA guidelines on high blood cholesterol have shifted the focus of therapy from the traditional LDL-C and non-high-density lipoprotein (non-HDL-C) targets to the intensity of statin treatment in various primary and secondary prevention categories.4 This shift in paradigm was heavily evidence- based, taking into consideration the results of major clinical trials impacting the outcomes of tens of thousands of patients. Nevertheless, when it comes to the individual patient who has suffered a coronary event, even high-intensity statin therapy may leave the patient with residual, often fatal cardiovascular risk that is not addressed by the guidelines.5 We believe that there should be an imperative to adopt more personalized lipid evaluation and treatment strategies to account for both traditional and non-traditional risk factors. The expectation is that a personalized approach may result in better individual outcomes.6
Our patient had established coronary artery disease and was aggressively treated to a target LDL-cholesterol level of less than 70 mg/dL, which was achieved on rosuvastatin 10 mg daily. Although his HDL-cholesterol and TG levels also showed marked improvement, with a seemingly optimal overall lipid profile, he returned with evidence of progression of his coronary artery disease, in addition to symptomatic carotid artery disease that necessitated intervention. He, therefore, was an ideal candidate for assessment of inflammatory markers and for advanced lipoprotein testing, as recommended by the NLA’s 2011 Expert Panel Statement on Biomarkers.7 A vertical auto profile (VAP) lipid panel was ordered; a normal high- sensitivity C-reactive protein (hsCRP) level was documented pre-therapy. Although levels of traditional risk markers were well controlled, an elevated Lp(a) level and LDL pattern B were identified as important residual risk markers8,9 and as potential targets for therapy with added niacin to improve this patient’s individual atherosclerotic risk.10,11
Niacin enjoys a long history as a lipid- lowering drug, with a multitude of mechanisms explaining its various anti- lipidemic effects. It inhibits a key enzyme for TG synthesis, resulting in increased hepatic ApoB degradation and decreased secretion of very-low-density lipoprotein (VLDL-C) and LDL-C particles.12 Niacin also partially inhibits adipose tissue lipolysis, thereby decreasing free fatty acid flux and hepatic VLDL synthesis. Furthermore, niacin decreases the removal of HDL by the liver, increases cholesterol efflux, and decreases the synthesis of Lp(a) without affecting its catabolism.13 Niacin, therefore, has been increasingly recognized as a “broad spectrum” lipid drug ever since its lipid-lowering effect was shown 60 years ago.14 Additional “pleiotropic” anti-inflammatory, antithrombotic, and antioxidant effects of niacin also have been suggested.15
Niacin is unique in its ability to significantly reduce plasma Lp(a) levels.16-18 The atherogenic potential of Lp(a) has been observed in several studies.8,19,20 Aggressive reduction of Lp(a) with lipoprotein apheresis by 60 to 70 percent, added to a background of optimal lipid therapy, resulted in a significant decrease in the annual rates of major adverse coronary events.21-23 The reduction of Lp(a) by niacin alone (at dosages of 1,000 mg to 2,000 mg daily) or in combination with a statin, however, is more modest and has ranged between 17 and 36 percent.13,16,24 Dramatic lowering of Lp(a) by 88 percent with niacin 2,000 mg daily in combination with atorvastatin 40 mg daily has been reported,10 a treatment similar to our reported case, in which we observed a 77-percent reduction in the Lp(a) level. Therefore, there seems to be a marked variability in the individual response to niacin therapy with respect to Lp(a) lowering. Niacin — alone and in combination with statin therapy — has shown favorable vascular effects, decreasing carotid atherosclerosis25 and carotid intima media thickness.26,27 Moreover, it has demonstrated mortality benefit, when given as monotherapy in high-risk men with high cholesterol, in the Coronary Drug Project.28 It is difficult to tease out the potential benefit of reducing Lp(a) levels, given the multiple salutary effects of niacin on other atherogenic lipid parameters.
Niacin ER did not demonstrate any improvement in major cardiovascular events when added to moderate intensity statin with and without ezetimibe in recent clinical trials. This strategy was tested in the Impact on Global Health Outcomes (AIM-HIGH) study29 and the Heart Protection Study 2 — Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study.30 The results have shaken the confidence in niacin in general among those who have used this as add-on therapy, as an HDL-C raising strategy.31 Despite these negative results, meta- regression derived from available clinical studies still supports the efficacy of niacin therapy in conferring atheroprotection and reducing cardiovascular disease risk.32 Of interest, the AIM-HIGH study showed that baseline and on-study Lp(a) predicted cardiovascular events in both the control and the niacin treatment arms, suggesting that Lp(a) still contributes to residual cardiovascular risk even after achieving target LDL-C levels with statin therapy.20 However, niacin treatment in the AIM-HIGH study resulted in only a modest 19 percent decrease of Lp(a) levels compared to placebo, which may have blunted the clinical effectiveness of niacin. It is important to point out that the placebo arm in the AIM-HIGH study included 50 mg of immediate-release niacin, to mimic the flushing effect of 1,500 mg to 2,000 mg of Niacin ER in the treatment arm, which resulted in a 4.2 mg/dL (11.8 percent) increase in HDL in the placebo group. Of note, very-low-dose niacin added to statin therapy previously has been reported to result in a modest, but significant, 5 percent (2.1 mg/dL) increase in HDL-C.33 Taking into consideration the significant 22 percent relative risk reduction in cardiovascular events seen in the Veterans Affairs High- Density Lipoprotein Intervention Trial (VA- HIT) study on gemfibrozil,34 with only 2 mg/dL (6 percent) improvement in HDL-C levels, leaves one to wonder if the AIM- HIGH study was, in reality, a comparison of high- versus low-dose niacin, and whether a portion of the anticipated benefit of niacin has already been realized in the placebo arm. In the HPS2-THRIVE study, 2,000 mg Niacin ER was combined with the anti-flush drug laropiprant, resulting in a 14 percent (6 mg/dL) increase in HDL-C; a similar effect previously was reported with such a combination.35 This, however, is lower than the 25- to 30-percent improvement in HDL-C observed with high-dose niacin without laropiprant36 and also reported in the AIM-HIGH study. Even though the HPS2-THRIVE study showed a nonsignificant improvement in the primary outcome of first major vascular events (nonfatal myocardial infarction, death from coronary causes, stroke or arterial revascularization), subgroup analysis was consistent with a significant 10-percent decrease in any revascularization procedure. The addition of laropiprant to niacin conceivably may have blunted the beneficial effects of niacin on optimally raising HDL-C and, perhaps, on the overall cardioprotective effect of niacin. It seems that neither trial answered the question with regards to the efficacy of adding high-dose, extended-release niacin alone (without laropiprant) to high-intensity statin therapy in improving outcomes compared with high-intensity statin therapy alone (without added low-dose niacin). Nevertheless, both trials do cast doubts on the effectiveness of niacin in reducing cardiovascular events in patients with atherosclerotic cardiovascular disease, despite significant improvements in HDL cholesterol and triglyceride levels, with the potential for increased risk of serious adverse events. Further careful subgroup analysis of both the AIM-HIGH and the HPS2-THRIVE studies may help shed further light on their overall unexpected negative outcomes.
Overall, niacin has a well-delineated side effect profile.37 Skin manifestations include a flushing sensation, which causes an average discontinuation rate of ≤ 6 percent for niacin ER, in addition to non-allergic skin rashes and dry skin. Patient education, dosage changes and use of moisturizing creams may alleviate many of these side effects and improve compliance. Significant liver toxicity is mostly seen with high-dose, slow-release preparation. Reversible niacin-induced insulin resistance usually has minimal effect on glucose control in diabetic patients and is rarely associated with new-onset diabetes. Except for a few case reports, niacin alone or in combination with statin therapy, in general, does not appear to cause or exacerbate muscle symptoms. Rare reported side effects of niacin include cystoid macular edema — causing blurred vision, nausea, and vomiting — and worsened peptic ulcers. Uncommon laboratory abnormalities include elevated prothrombin time and uric acid, and decreased platelet count and serum phosphorus.
Our patient was gradually titrated on extended-release niacin to 2,000 mg daily, which he tolerated well with only minimal flushing symptoms at the beginning of the therapy. He did not complain of any muscle symptoms, and his laboratory data, including his liver function tests and A1c, remained normal. His initial Lp(a) was moderately elevated at 26 mg/dL and decreased to 22 mg/dL on rosuvastatin alone. However, after the addition of niacin and a change from rosuvastatin to atorvastatin (as a result of formulary considerations), his Lp(a) dramatically decreased to a safer level of 5 mg/dL, and his LDL pattern improved from the highly atherogenic pattern B to a favorable, less atherogenic pattern A. Overall, he has done well, without evidence of progressive atherosclerosis for the three years he has been on this combination therapy. His response to niacin, with regards to the lowering of Lp(a), was more dramatic than previously reported in the major clinical trials discussed earlier, the type of response associated with clinical improvement seen in lipoprotein apheresis studies. It is plausible that our patient’s carotid artery disease may have antedated the initial start on statin therapy to where the CEA may have been inevitable. However, the appearance of symptoms related to his carotid artery stenosis and the evidence of progression of his coronary disease while on statin therapy were concerning for untreated residual risk.
Overall, this underscores the need for earlier detection and primary preventive treatment efforts to avoid the development of cardiovascular disease.
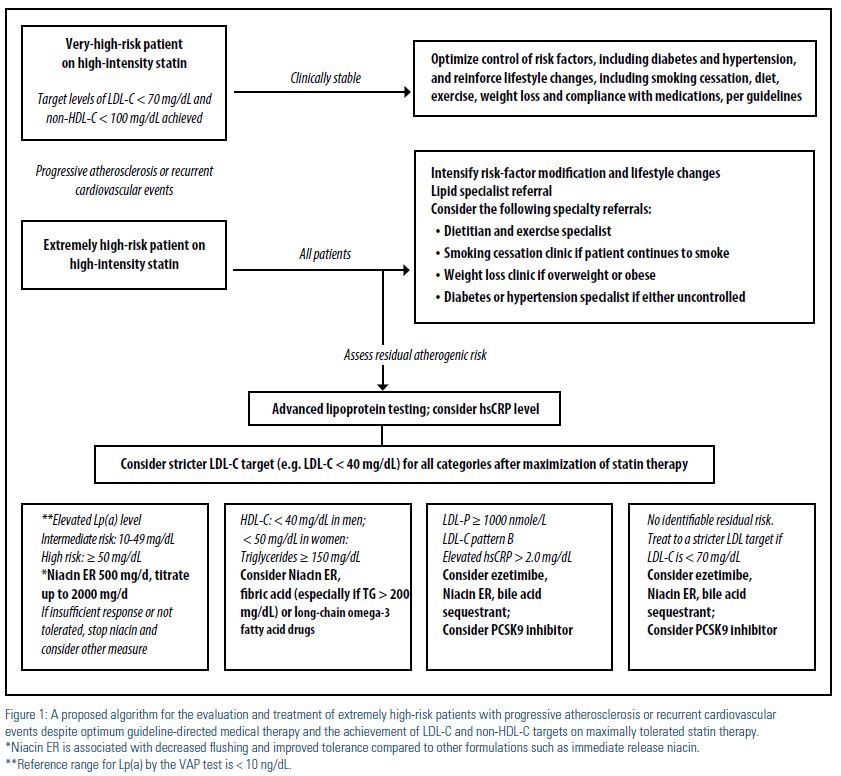
The NLA Recommendations for Patient- Centered Management of Dyslipidemia6 provide clearly defined criteria for atherosclerotic cardiovascular disease (ASCVD) risk assessment, treatment goals for atherogenic cholesterol, and levels at which to consider drug therapy in four risk categories: low, intermediate, high, and very high. However, when it comes to treatment of patients with progressive atherosclerosis or recurrent events, despite evidence-based therapy, as in our patient, the NLA Expert Panel consensus’ view is that “very aggressive therapy to lower atherogenic cholesterol levels to values well below goal thresholds may be considered for such patients, although it is acknowledged that this approach is not clearly supported by clinical trial evidence.” We propose that such patients be categorized as extremely high risk, and undergo specialized patient-centered care by a lipid specialist for detailed analysis and treatment of their residual atherogenic risk, as shown in Figure 1. It is imperative to understand that large, randomized clinical trials, which form the basis of much of our evidence-based medicine, have inherent problems38 when applied to an individual patient with unique characteristics and residual risk not addressed by such trials. A patient- centered and personalized approach to the interpretation and application of multiple studies as they pertain to a single patient is the crux of individualized medicine, making every patient’s unique response essentially a one-patient clinical trial.39
In conclusion, when an optimally treated patient with cardiovascular disease has another cardiovascular event, it is reasonable to consider treatment options which have not been strongly endorsed by any guidelines. Such options, however, are not considered standard of care due to the absence of sufficient evidence, and patients should be counseled accordingly. If dramatic lowering of Lp(a) or other atherogenic particles, beyond what has been demonstrated in the major negative randomized clinical trials, is not achieved with niacin, then the clinician should heed the results of such trials and consider stopping niacin. Addition of ezetimibe to statin therapy may be another option to further lower LDL cholesterol levels and improve cardiovascular outcomes, as demonstrated in the IMPROVE-IT trial.40 Aggressive LDL-C lowering with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors has recently emerged as another lipid-lowering therapeutic option in patients inadequately controlled on maximally tolerated statin therapy; however, outcomes data are pending.41 Beyond the currently available potent LDL-lowering therapies, which may have reached their maximum potential to reduce risk, there has been interest in raising HDL with cholesteryl ester transfer protein (CETP) inhibitors. However, despite the significant increase in HDL-C with the CETP inhibitor dalcetrapib in patients who had a recent acute coronary syndrome, there was no reduction in the risk of recurrent cardiovascular events.42 Renewed interest has recently surfaced, however, in this therapy’s potential benefit on cardiovascular outcomes in specific pharmacogenetic variants.43
Disclosure statement: Dr. Kolakalapudi has no disclosures to report. Dr. Omar has no disclosures to report.
References are listed on page 32 of the PDF.





.jpg)
.png)