William Hazlitt, an 18th century literary critic and philosopher, once said, “When a thing ceases to be a subject of controversy, it ceases to be a subject of interest.”1 If one accepts his premise, then interest in high-density lipoprotein (HDL) shall endure for some time. As far as contemporary medical controversies go, the HDL story is definitely in contention.
Without question, our evolving understanding of the structure and metabolism of lipoproteins has led to important insights into treatment and prevention of coronary heart disease (CHD). Results from these efforts are reflected in favorable trends in national lipid and lipoprotein levels.2 Indeed, due in part to improved nutritional and clinical guidelines, increased public awareness of risk factors and new lipid-lowering therapies, CHD mortality has leveled off and even declined since the late 1960s.3
These achievements, along with a growing number of promising therapies that lower low-density lipoprotein (LDL), elicit a sense of optimism that more advances will follow.4 However, CHD stubbornly persists in the U.S. as a leading cause of death in both men and women — a sobering reminder that this medical success story is still a work in progress. Thus, the goal of curtailing CHD remains paramount and still seems far off.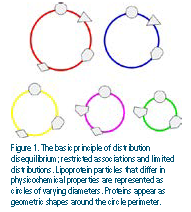
Interventional clinical trials are considered the ultimate test and final arbiter for the validity of scientific theory in medicine. Conducting a clinical trial exemplifies a conviction that one has arrived at a fundamental understanding concerning the pathophysiology of a disease and a confidence that a deliberate action taken will favorably change the outcome. Thus, it was widely anticipated that therapeutic increases in HDL-cholesterol (HDL-C) would translate into coronary artery disease (CAD) protection to augment the already successful approach of lowering LDL-cholesterol (LDL-C). However, the failures of HDL-C-raising therapies — including three cholesteryl ester transfer protein (CETP) inhibitors5 and extended- release niacin6 — have struck at the very core of a favored scientific philosophy.7
Some scientists, clinicians, and policy makers have begun to write off HDL as a therapeutic target or biomarker. Still, there are many who passionately argue that the pendulum has swung too far in the opposite direction and insist that HDL still holds great promise. HDL has indeed become controversial — and more interesting than ever.
Did the clinical trials mentioned above really kill HDL or did they simply kill the myth that HDL-C is a good marker for HDL? A more sobering assessment of these clinical trials reveals that they were never designed to assess the health benefit of HDL or even test whether modulating HDL would improve CHD outcomes. Instead, they were a referendum on the ability of HDL-C to reflect improved functionality of all HDL particle populations. The notion that HDL and HDL-C are one in the same — or even biologically equivalent — has been impugned.8 It is becoming clear that viewing HDL biology through a cholesterol lens and the fallacy of a “one-component- represents-all” approach have reached the end of their usefulness.
Moving away from a reductionist model and toward a high-definition systems approach to particle population monitoring is inevitable. This is a key tenet of precision medicine (PM), and translating this approach to atherosclerotic cardiovascular disease brings with it the most viable means to advance diagnosis and treatment. This may appear obvious to some, but there is scant evidence that this strategy has been broadly adopted. Of the ~13,000 articles identified using the search term “personalized or precision medicine,” only 0.6 percent can be linked to “atherosclerosis or heart disease.” This is in stark deference to the cancer field, which accounts for ~36 percent of these publications, and is not unexpected given that oncology researchers engaged the PM model early on.9 The similarities between cardiovascular disease and cancer are evident, including an underlying genetic susceptibility influenced by non-modifiable and modifiable risk factors. Perhaps some valuable lessons may be gleaned given the dramatic increase in approved cancer drugs in the past decade.10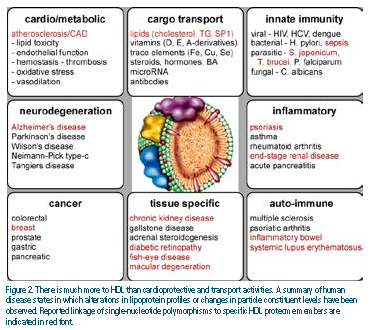
The limitations of the cholesterol-centric focus have been glaringly revealed by recent advances in mass spectrometry that have uncovered an expanded lipoproteome and lipidome unforeseen just a few years ago. As a lipoprotein class, HDL has the most extensive particle diversity and population heterogeneity, with more than 100 proteins11 and ~200 lipid species12 in an undetermined number of combinations.13 Particle constituents exist in distribution disequilibrium with each other and to the particle population as a whole, which can be observed across multiple separation methods.14-16 Figure 1 illustrates this model from the perspective of the HDL proteome, but this also is true for specific lipid species, as well.17
There is an intrinsic relationship between particle constituents, physicochemical properties, and functionality. Each particle is a macromolecular assemblage of molecular species that produces a related set of physicochemical properties such as density, size, or electrophoretic mobility. However, consider the idea that each particle is a collection of constituents that are selectively combined to generate a self- contained set of “operating instructions” that dictate the particle’s activities. Although separating particles based on physicochemical properties has allowed one to apportion activity to particle subfractions,18 one needs to look beyond defining HDL subpopulations based on their physicochemical attributes.19 Instead, it is the protein constituents themselves that should serve as surrogate markers to classify HDL particles and provide the basis for assigning functionality.20
The most essential facet of a healthy HDL profile is the capacity of the particle populations to perform their biological function(s) as effectively and efficiently as possible. This notion of HDL functionality is embodied in the term “HDL quality,” which encapsulates the atheroprotective properties of HDL. Those properties include reverse cholesterol transport and its antioxidant, anti-inflammatory, and antiapoptotic activities, among others.21
HDL functions also can become impaired or “dysfunctional,” thus complicating the picture.22 Particle alterations that reshape functionality are a result of genetic and metabolic factors that directly modify the lipoproteome and lipidome as well as influence particle population dynamics.23
It is no surprise that HDL exhibits a range of activities outside the traditionally viewed roles in cardiovascular disease.24
These disease/function associations are mirrored in the proteome, with numerous constituents playing known roles in various other diseases.25 Figure 2 summarizes a variety of diseases in which alterations in lipoprotein distribution profiles or changes in HDL particle constituent levels have been observed in humans.
Additionally, genetic association studies in diseased individuals have identified single- nucleotide polymorphisms in specific HDL proteome members, although confirmatory studies with independent cohorts are still required to validate some of these associations. Yet the model of subparticle functional heterogeneity predicts a role for HDL in distinct biological processes. Some of these involve important roles in cardioprotection — others may not. It also follows that, if we can identify these subspecies or markers of these subspecies and relate them to disease protection or progression, we will have a much better biomarker with which to track disease and stratify risk in individuals.
We contend that the current HDL measurements are fundamentally insufficient to address the challenge posed by particle diversity and heterogeneity.
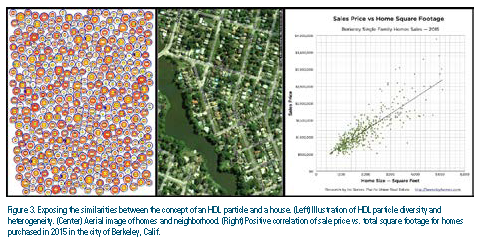
A simple analogy is presented in Figure 3 to make the point. An illustration of HDL particle diversity and population heterogeneity is shown (Figure 3, left image). Each is a symbolic rendering of the proteome and lipidome. No two particles are identical, but some molecular similarities can be discerned. For example, the cholesterol:triglyceride ratio is reflected as a color gradient from red to orange and at the center of each particle is a proteomic core comprising apolipoprotein combinations. Juxtaposed to the particle population is an aerial view of a neighborhood (Figure 3, center image). Even without closer inspection, one knows that each home has distinct characteristics that make it unlike another. It is obvious that the combination of features that make a home unique also contributes to its resale value. However, one could simply choose one factor, such as the home’s total area, as the only criteria needed to make a purchase, confident that this is an essential measure of the home’s value. In fact, there is a significant positive correlation between sale price and square footage, as illustrated in the right image in Figure 3. Yet, trusting this one factor demonstrates an indifference to determining the home’s real value. Only through a careful inspection of the property can all the home’s qualities be assessed as a reasonable means to make the comparison with other purchase possibilities. The parallels to considering HDL “quality” should be apparent. The value is determined by details. Just as one probably should not buy a home simply based on total square footage, one should avoid relying on HDL-C when considering the health benefits of HDL.
It has been suggested that “advanced lipid testing” techniques, which measure particle number or distinguish particle subpopulations using physicochemical attributes such as density, size, or electrophoretic mobility, bring diagnostic improvements. HDL subtypes classified in this manner result in a highly constrained particle nomenclature.26 Defining HDL as having three, five, or even 13 particle subtypes may offer incremental insight, but we argue that these still are inadequate to address the problem at hand. Particle modeling based on combinations of distinct protein and lipid constituents are predicted to be significantly larger.
The age of Omics Research is now offering a glimpse at the horizon of lipoprotein characterization and, perhaps, a truer understanding of the requirements for HDL molecular profiling. In an ideal world, it would be best to develop a phenotyping strategy that is capable of measuring large numbers of particles and all of their associated constituents. As this likely involves tens of thousands of particles in a drop of plasma, this technologically is out of reach. However, next-generation HDL measurements will need to strike a balance between clinical assay practicality and a breadth of particle coverage that allows one to monitor numerous particle subpopulations, or at least their surrogate markers, that are relevant to a particular disease state. Entry points to this area are emerging as, for example, Sacks and colleagues have begun to fractionate HDL using immunoaffinity separation techniques and relate these to disease risk.27 While such candidate particle approaches have promise, one ultimately would like to develop technologies that track the entire HDL “interactome”28 in an unbiased way. Once translated into clinically feasible assays, such measures would be applicable to contemporary concepts such as precision medicine.
Sophisticated molecular phenotypes derived from HDL diagnostic tests that rely on integrated biomarker panels will go hand-in-hand with the genomic sequencing efforts. The lack of molecular context and granularity that connects physiological consequence to the sequence variants identified29 remains a crucial element to designing future interventional strategies. Most excitingly, their use to advance the study of the progression of HDL-related diseases such as CHD, assess efficacy of experimental therapeutics, and stratify patients into groups that may respond better to certain treatments should not be opportunities missed. Given the stunning compositional complexity of HDL and the widely varying known functionalities of the proteins that associate with the particles, we offer the belief that HDL is a nexus that mediates a tremendous amount of unknown biology. Staggering resources have been poured into raising HDL-C. Shouldn’t we invest a bit more to better track the protein combinations that likely mediate these beneficial activities and enable assessment strategies that guide treatment for individuals rather than the population?
Disclosure statement: Dr. Altmann owns HDL Apomics LLC. Dr. Davidson has received speaker and consulting honoraria from Eli Lilly, and he is on the advisory board of HDL Apomics.
References are listed on page 35 of the PDF.






.jpg)
.png)













Comments
HDL Resources for researchers and clinicians is in the works
We are working on the construction of an HDL resource site to aid researchers and clinicians interested in HDL. Still building content and preparing the bioinformatics. I will let you know when we will begin to make the websit available to the public.
Thanks