The global epidemic of type 2 diabetes (T2DM) continues unabated. Atherosclerotic cardiovascular disease (ASCVD) and heart failure (HF) are the most common causes of T2DM-related mortality. More than 90 percent of patients with T2DM are overweight or obese. The pathogenesis of this disorder is heterogenous, but insulin resistance and relative β-cell dysfunction are its key characteristics.
Insulin resistance is a major contributor to cardiometabolic syndrome, comprising the characteristic atherogenic dyslipidemia — triglyceride-rich lipoproteins, elevated apolipoprotein B (apo B), and low high-density lipoprotein cholesterol (HDL-C) — hypertension and increased thrombogenecity. Over the course of years, β-cell loss progresses, partly because of increased insulin demand resulting from ongoing insulin resistance, which worsens by a net caloric excess and failure to achieve appreciable weight loss, thus creating a vicious circle. Not surprisingly, while lifestyle changes such as diet and exercise may initially control hyperglycemia, most patients over the years will require various drugs designed to improve insulin resistance or β-cell function, often in combination as double or triple therapy and, ultimately, as insulin replacement.
Metabolic and Vascular Effects of Insulin
Insulin has both metabolic and mitotic effects at the cellular level. Under physiological circumstances, insulin — via phosphoinositide 3-kinase (PI-3 kinase) and mitogen-activated protein kinase (MAP- kinase) pathways, respectively — has both anti-atherosclerotic and anti-inflammatory actions, e.g. increased nitric oxide synthase, reduced nuclear factor — kappa B NF-κ B, reduced platelet aggregation, etc., as well as pro-atherosclerotic/pro- inflammatory actions (e.g., increased plasminogen activator inhibitor 1 [PAI-1], increased endothelin-1 [ET-1] receptor activity, increased cell proliferation, etc.) in the vessel wall. The former effects likely predominate in the absence of insulin resistance.1-3 Because of variable degrees of insulin resistance, some patients will require a progressively increasing insulin dosage to overcome the underlying defects in insulin action. However, at the clinical level, it is not clear if a subset of such patients requiring a very high insulin dosage to overcome their insulin resistance may actually have deleterious effects, particularly from the cardiovascular viewpoint. 
Potential “insulin-induced harm” was the theme of the recent provocative perspective articles by Nolan, et al.4,5
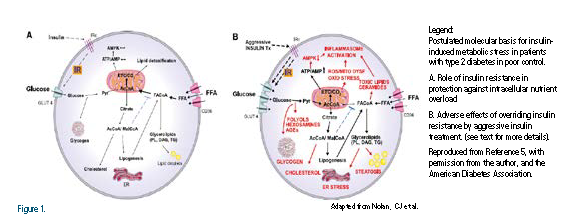
They propose that, in T2DM patients with unremitting obesity primarily the result of caloric surplus and a lack of physical activity, the worsening insulin resistance may actually reflect an adaptive defense mechanism against “insulin-induced metabolic stress.” They also propose that the induction of insulin resistance serves as a “safeguard” against insulin- induced glucolipotoxicity at the level of myocardium, in particular (Figure 1). They discuss the deleterious role of intensive insulin regimens in driving excessive entry of glucose and free fatty acids (FFA) in the myocardium, thus overloading the electron transfer chain and leading to mitochondrial dysfunction, excessive reactive oxygen species (ROS) production, and oxidative stress.4 These phenomena and failure to oxidize FFA result in the accumulation of toxic lipids (e g. ceramide) and reduced Adenosine Monophosphate Kinase (AMPK) activity, leading to the activation of inflammasomes and further myocardial injury. The increased prevalence of cardiomyopathy in patients with T2DM has been recognized since the Framingham Heart Study.6 It is likely that such patients account for the increased risk of heart failure in patients with diabetes.
Table 1 summarizes the various mechanisms for adverse cardiovascular effects of intensive insulin therapy in patients with refractory hyperglycemia.
Interpreting Insulin Effects in Clinical Trials
The benefits of intensive glycemic control, with or without insulin therapy, on microvascular complications of both types 1 and 2 diabetes are well established in landmark trials such as the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS), respectively.7,8
However, none of the clinical trials with intensive insulin therapy in obese T2DM patients thus far has shown a reduction in CVD mortality. The UKPDS trial is the only primary prevention trial that provided evidence of significant but modest benefits from “intensive” glucose control (with insulin, with or without sulfonylurea therapy). However, CVD outcomes and mortality benefits were seen only after 10 additional post-trial years of follow-up.9
Of note, these patients were relatively younger, newly diagnosed and, on average, overweight but not obese at baseline (mean age 53 years, BMI 28), and the mean insulin dose was only 22–36 units daily.
In contrast, three large cardiovascular randomized controlled trials (RCTs) compared intensive and conventional glycemic treatment. These included Action to Control Cardiovascular Risk in Diabetes (ACCORD),10 Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE),11 and the Veteran Affairs Diabetes Trial (VADT).12 The trials involved >23,000 older patients with a mean age between the three trials of 60 to 66 years, a mean BMI of 28 to 32, and a mean duration of diabetes from 8.0 to 11.5 years. Of those, between 32 and 40 percent had pre-existing CVD. A particular concern was an unexplained 22 percent increase in total deaths and a 35 percent increase in CV deaths in ACCORD, despite a reduction in ischemic coronary events.10,13 In VADT,12,14 a significant decline was reported for major CV events, but not total deaths, after a median of 9.8 years of observational follow-up.14 In ADVANCE,11 a reduction in total deaths or CV events was not seen even after 5.4 years of additional follow-up.15 In both ACCORD and VADT, intensive insulin therapy was associated with considerable weight gain and increased rates of severe hypoglycemia. During both trials there was a significant increase of 35 and 32 percent in CV mortality, respectively.10,12
Thus, in these pivotal trials, intensive insulin therapy in relatively older patients with a long duration of pre-existing disease, including pre-existing CVD in many, was shown to adversely affect cardiovascular mortality and total mortality, despite a reduction in ischemic events.
A few other trials have investigated the long-term effects of insulin compared to conventional therapy in patients with T2DM and CVD. The Diabetes Insulin- Glucose in Acute Myocardial Infarction study (DIGAMI-1) was a RCT in which 620 patients with acute myocardial infarction (MI) were randomized to short-term intensive insulin or conventional therapy.16
There was an 11-percent decrease in mortality at 3.4 years. However, none of these patients was on statin therapy
at baseline; thus, the implications of the results are unclear. DIGAMI-2 (N=1,253),17 and the Hyperglycemia: Intensive Insulin Infusion In Infarction (HI-5) study (N=240),18 showed no significant effects of intensive insulin on mortality in post-MI patients after mean periods of three years and six months, respectively. However, in an observational follow-up of DIGAMI-2, there was a marked increase in the composite of re- infarction, stroke, and total mortality (HR 1.78, 95-percent confidence interval, CI [1.14-2.40]).19
In the Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus (HEART2D) trial, a prandial insulin regimen was compared to basal insulin.20 During a mean follow up of 2.7 years, there were no differences in CVD outcomes or glycemic control. Finally, the Outcome Reduction with an Initial Glargine Intervention trial (ORIGIN trial) — in >12,000 patients at high risk for CVD with recent onset of T2DM or pre-diabetes — compared basal insulin glargine or noninsulin treatments.21 The baseline hemoglobin A1c (HbA1c) was relatively low at 6.4 percent, and the mean daily insulin dose was only 28 units. There was no effect on CVD outcomes (HR, 1.02; 95 percent CI, 0.94-1.11) after a median follow-up of 6.2 years.
The Implications in Type 2 Diabetes Management
Nolan, et al., present a persuasive scientific rationale for the detrimental cardiovascular effects of long-term treatment with an increasing insulin dosage in a subset of obese patients with T2DM, where insulin resistance may protect the heart and other organs. Their hypothesis may explain the lack of expected cardiovascular benefits in such patients where insulin-induced metabolic stress may dampen the effects of attempted intensive glycemic control with an increasing insulin dosage. They make a critical distinction between overriding insulin resistance via increased insulin availability versus nutrient off-loading.
For example, in the long-term Action for Health in Diabetes (Look AHEAD) trial, the lifestyle program in subjects with mean BMI of 36 resulted in a significant weight loss, and appreciable trends in reductions in mortality (HR 0.85, 95 percent CI 0.69-1.04), as well as fatal and non-fatal MI, and HF (22). Furthermore, in the recent CVD outcome EMPA-REG trial, with the sodium glucose-cotransporter-2 (SGLT-2) inhibitor empagliflozin, in >7,000 T2DM patients there was a 14-percent reduction in the primary endpoint (CV deaths, nonfatal MI, or nonfatal stroke) (P=.04), a 32-percent reduction in all-cause deaths (P<.001) and a 35-percent reduction in hospitalization for heart failure (P=.002).23 These benefits were accompanied by modest reductions in HbA1c levels, body weight (~2 kg), waist circumference (~2 cm), and systolic blood pressure (~4 mm) in the absence of hypoglycemia, in contrast to the intensive insulin trials discussed above. Recurrent hypoglycemia is both mechanistically and epidemiologically linked with increased CV mortality in recent studies.24,25
Unfortunately, there currently are no pharmaceutical agents that would effectively and safely improve insulin sensitivity without causing further weight gain. Thus, testing the Nolan hypothesis, at least in patients with severe obesity and poor control despite increasing insulin dose (e. g >1-2 units/Kg body weight) would be of much interest. Therefore, a clinical trial comparing an insulin regimen with a combination of strategies that reduce insulin resistance, reduce weight, and avoid hypoglycemia such as intensive lifestyle changes + SGLT2 inhibitor + GLP-1 agonists and perhaps an alpha-glucosidase inhibitor is needed to assess the CVD outcomes. Bariatric surgery is another alternative,26 but the cost and long-term adverse effects need consideration.
Disclosure statement: Dr. Ganda has received research support from Amarin Pharmaceuticals and consulting fees and speaker honoraria from Merck, Sanofi, and Amgen.
References are listed on page 35.






.jpg)
.png)











