It is well-established that, compared to men, post-menopausal women enjoy a degree of protection against atherosclerotic cardiovascular disease (ASCVD).1 The rate of ASCVD in post-menopausal women matches or even exceeds that in men.1 In spite of the pre-menopausal protection for women, ASCVD remains a major factor in morbidity and mortality in women and is, as it is for men, the largest single cause of death in women worldwide.2
Gender differences in the occurrence of ASCVD have long intrigued investigators because these differences might provide an opportunity to understand the mechanisms of the disease. Many risk factors for ASCVD, such as plasma lipids and lipoprotein levels, fibrinolytic factors and insulin resistance, are responsive to regulation by sex hormones and display changes after menopause that are associated with increased cardiovascular risk.3,4,5 Another lipid risk factor that may be relevant to the changing ASCVD risk profile in women is elevated plasma concentrations of lipoprotein(a) [Lp(a)].
In this editorial, we shall tackle the three key questions regarding Lp(a) and ASCVD in women: (1) Is elevated Lp(a) truly an ASCVD risk factor in women? (2) Does Lp(a) contribute to the changes in risk that accompany menopause? (3) Is there a case for measuring Lp(a) concentrations in women and for therapeutic management of Lp(a) to lower risk of ASCVD events in women?
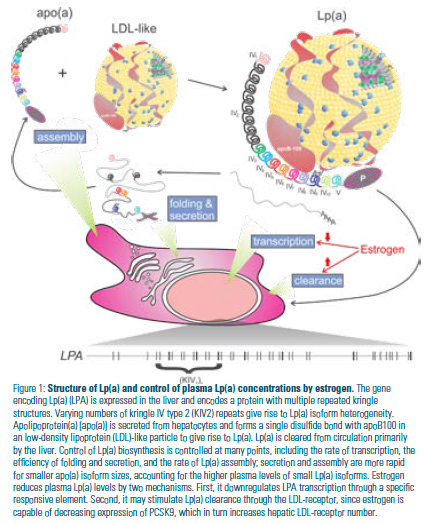
Lp(a) closely resembles low-density lipoprotein (LDL) but is distinguished by the presence of the large glycoprotein apolipoprotein(a) [apo(a)] that is covalently linked to apolipoprotein B100 (apoB100) (Figure 1). Plasma concentrations of Lp(a) vary widely (~1,000-fold) in the human population while being largely stable within an individual during their lifetime.6 Lp(a) levels are genetically determined, with most of the variation attributable to differences in the gene encoding apo(a). (Figure 1).6 The size of Lp(a) varies because of differences in the size of alleles of apo(a) that encode proteins with different numbers of type 2 kringle IV repeats. There is an inverse correlation between the allele size of LPA (the gene encoding apo(a)) and Lp(a) concentrations in plasma.6
Studies consistently have reported that plasma Lp(a) levels rise after menopause and that this rise can be attenuated by hormone replacement therapy (HRT).13,14 This prompts the question of whether Lp(a) contributes to the increase in ASCVD risk that is observed in post-menopausal women. The extent of increase in Lp(a) is relatively modest in most studies, with an approximately 20% to 30% increase observed.14 However, the largest cross- sectional study seeking to answer this question found that adjustment for age reduced the increase to a non-significant 8%.15 Because other ASCVD risk factors also increase after menopause, it is difficult to determine whether an increase in Lp(a) levels is a direct culprit in post- menopausal ASCVD risk. Age itself is a risk factor because atherosclerosis is a progressive disease with effects that accumulate throughout adulthood. While it has been shown that elevated Lp(a) is an independent risk factor for first and recurrent coronary artery disease (CAD) in post-menopausal women,16,17 no study has directly compared the risk attributable to Lp(a) in pre- and post-menopausal women. Although we might speculate that Lp(a) plays less of a role in pre-menopausal women, it also could be argued that genetic risk factors such as elevated Lp(a) would be over-represented in younger women.
Estrogens are thought to lower Lp(a) through a direct effect on transcription of LPA,18,19,20 and possibly through an effect on Lp(a) clearance by raising levels of the LDL-receptor in the liver (Figure 1).21,22,23 HRT reduces Lp(a) concentrations by 20% to 25% in most studies.14 Interestingly, transdermal HRT has no effect on Lp(a), while oral HRT does.24 These reductions might be sufficient to yield a reduction in ASCVD events, if sufficient numbers of patients were treated. The extent to which Lp(a) must be lowered to see an effect is not entirely clear, however, because outcome studies of Lp(a)-lowering therapies have not been performed. In the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/ High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) trial of extended-release niacin, Lp(a) levels were reduced by 21% compared to placebo, but no reduction in ASCVD outcomes was reported.25 The reasons for this are obscure because niacin generally failed to demonstrate efficacy in reducing ASCVD events despite having several favorable effects on the lipid profile.26
Two studies of HRT provided tantalizing hints of an interaction between HRT and Lp(a) levels in ASCVD events. Shlipak, et al., found that HRT appeared to be more effective in reducing events in women with higher initial Lp(a) levels.17 Suk Danik, et al., found that HRT eliminated the excess ASCVD risk attributable to elevated Lp(a).16 However, neither of these studies were randomized controlled trials (RCTs) and, therefore, could not directly address the question of whether lowering Lp(a) with HRT reduces ASCVD risk. Since HRT of this type is no longer recommended for cardioprotection in post-menopausal women – in fact, it has been shown to increase risk27,28 – it will not be possible to follow up on these observations.
In addition to estrogens and progestins, other compounds have been investigated for their effects on Lp(a) levels. Inconsistent findings have been reported about the effects of selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) on Lp(a) levels,24 although meta-analyses did manage to demonstrate Lp(a)-lowering effects of the SERMs raloxifene and tamoxifen.29,30 A recent RCT of the AI letrozole in post- menopausal women with breast cancer showed an approximate doubling of Lp(a) levels with this compound,31 although it is difficult to disentangle the effect of the AI from the consequences of withdrawal of the subjects from tamoxifen, which occurred just prior to study entry. Tibolone is a synthetic steroid with estrogenic, progestogenic and androgenic activity; most studies, including meta-analyses, have revealed this compound to have an Lp(a) lowering effect.24,32
While current guidelines do not support screening for elevated Lp(a) in the general population,33, 34,35,36 the prevalence of this risk factor suggests that fully 20% of the population possesses Lp(a) levels sufficiently high (>50 mg/dL) to increase their risk of ASCVD more than 50%.9 Current guidelines suggest measuring Lp(a) in patients at high risk for, or with existing, ASCVD.36 There is no specific recommendation regarding female patients, either pre- or post-menopause. In terms of the clinical management of patients with elevated Lp(a), there is no therapy that currently is approved specifically for the lowering of Lp(a). It is recommended that modifiable risk factors be treated more aggressively in such patients.36 In general, although Lp(a) concentrations might be lowered by HRT and SERMs, these should not be considered first-line interventions in post-menopausal women with elevated Lp(a). Certain drugs – such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and, possibly, cholesteryl ester transfer protein (CETP) inhibitors – are able to lower Lp(a), in addition to other beneficial effects on the lipid profile.37,38 An Lp(a)-specific antisense oligonucleotide therapy that targets apo(a) expression is in clinical trials and can remarkably lower Lp(a) concentrations.39
It is exciting to contemplate how this compound finally will allow the idea that lowering Lp(a) reduces risk for ASCVD to be directly tested. Such studies also will allow the question of the role of Lp(a) in cardiovascular risk in women, both pre- and post-menopause, to be resolved.
Disclosure statement: Dr. Boffa has received honorarium from IONIS and Dr. Koschinsky has received honoraria from Amgen, Eli Lilly and Pfizer, as well as research grants from Eli Lilly and Pfizer.
References available here.





.jpg)
.png)












