A 73-year-old woman with coronary and peripheral arterial disease with hyperlipidemia was referred for a lipidology consult because of elevated liver function tests (LFTs) while on rosuvastatin. Aspartate amino transferase (AST) rose from a baseline of 26 to 120 and alanine amino transferase (ALT) from 15 to 132. She did not drink alcohol nor take other hepato-toxic pharmaceuticals. An abdominal ultrasound showed normal liver parenchyma, a 7 mm hepatic cyst and a post-cholecystectomy pattern. The patient was instructed to discontinue rosuvastatin prior to the referral appointment. She also presciently discontinued her weight-loss nutraceuticals (caralluma fibriatta, lean green tea and platinum garcinia). At the time of her consultation, her liver function tests (LFTs) had returned to baseline. She was recommended to remain off the nutraceuticals and re-challenged with rosuvastatin. Repeat LFTs at 1, 2, 3 and 6 months (Figure 1) post-statin re-challenge remained normal while off the nutraceuticals.
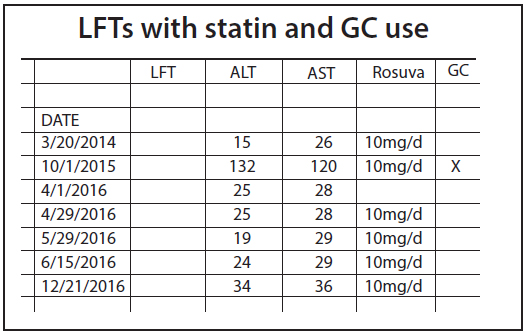
Figure 1.
Lipidology referrals for elevated LFTs often impugn statin medications as the cause. This is perfectly reasonable when the LFT elevation is temporally related to initiating cholesterol-lowering therapy. However, as seen in this case, other sources may be involved.
In 2012, the U.S. Food and Drug Administration (FDA) recommended LFTs be performed prior to initiating statin therapy to obtain a baseline measurement and to avoid starting statins in the presence of severe liver dysfunction. AST and ALT were recommended for baseline assessment. Repeat LFTs were recommended only when clinically indicated. Others elect to obtain gamma-glutamyl transferase (GGT), total protein, albumin or bilirubin when appropriate. The FDA discouraged repeated LFT monitoring without clinical evidence of liver dysfunction. However, LFTs still are – not infrequently – obtained to monitor statin therapy or for an unrelated reason. In these instances, elevated LFTs may be detected. FDA and statin labels recommend re-assaying elevated LFTs, then continuing, holding or discontinuing statin therapy when AST or ALT are greater than three times the upper limit of normal.
LFTs may increase for a number of reasons other than statin therapy (Figures 2,3). Even so, when LFTs increase significantly, it is reasonable and advised to temporarily discontinue any medication with a potential impact on liver function and medicines which are metabolized by the liver and may thus, not be cleared efficiently. Once elevated LFTs return to baseline, statin therapy may be re-challenged, while monitoring LFTs.
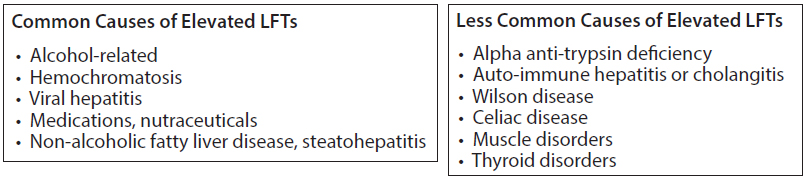
Figure 2 & 3
Our patient self-administered Garcinia cambogia (GC) a weight-loss supplement readily available over-the-counter. GC (also known as brindleberry, malabar tamarind, and kudam puli [pot tamarind]) is extracted from the green, pumpkin-like fruit of the garcinia gummi gutta, a tropical tree of Indonesia.
GC is a source of hydroxycitric acid (HCA), which is known to inhibit oxaloacetate and acetyl CoA, interrupting lipogenesis in vitro. However, GC failed to achieve a significant weight loss over 12 weeks compared to double-blinded placebo in those on a high-fiber, low-energy diet.1 A meta-analysis of nine studies found a small, short-term weight loss that was not statistically significant when only rigorous randomized clinical trials were analyzed.2
The FDA warned in 2009 of liver damage from Hydroxycut®, a poly-herbal containing GC, which was removed and recalled from the market. Hydroxycut® later was reformulated without GC and re-introduced to the market. In 2017, the FDA removed Fruta Punta Garcinia Cambogia PremiumTM from the market because it was tainted with sibutramine. Previously, the FDA had removed sibutramine (Meridia®) from the market as a prescription weight-loss drug because “it may increase blood pressure and/or heart rate in some people and may present a significant risk for people with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke.”3 Cases have been reported of patients developing “Serotonin Syndrome” when combining their serotonergic medicines with Garcinia cambogia.4 GC is contraindicated when using selective serotonin reuptake inhibitors (fluoxetine, paroxetine, escitalopram, sertraline), tricyclic antidepressants, dextromethorphan, pentazocine and tramadol.
Some claim other herbal weight-loss compounds are more likely the cause of hepatic inflammation in the Hydroxycut® controversy.5 A case of habitual high-dose green tea use, which our patient also took, also has been reported as a source of elevated LFTs.6 The common practice of combining numerous supplements into one preparation obscures the source of side effects when they do occur.
However, more than 25 cases of severe chemical hepatitis associated with GC have been reported, one requiring liver transplant.7,8 Even with FDA cautionary warnings, GC continues to be promoted through hundreds of distributors for weight loss, fat and calorie burning, increased energy and non-stimulant diet support. Guidance for monitoring GC use is lacking, but evaluation of LFTs seems prudent. It is important to report to the FDA any elevated LFTs or liver failure associated with supplement use, especially GC.
In the unregulated environment of nutraceuticals, supplements that appear harmless to our patients may have untoward consequences. Statins often bear the blame for symptoms or findings from other sources. When assessing elevated LFTs, investigation of supplement use and excluding other causes may vindicate statin use.
Disclosure statement: Dr. Trippi has received honorarium from Sanofi, Amgen, Akcea, Kastle, Pfizer, and Amarin.
References available here.






.jpg)
.png)











