The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial was a randomized trial in 27,564 patients with stable atherosclerotic cardiovascular disease (ASCVD), evaluating evolocumab vs placebo added to statin therapy.(1) The hazard ratio for reduction of the primary endpoint (composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina or coronary revascularization) and the key secondary endpoint (composite of cardiovascular death, myocardial infarction, or stroke) was 15% and 20% respectively. Since the trial was published a number of subgroup analyses have been performed, with interesting results that will be briefly summarized here.
The Evaluating PCSK9 Binding Antibody Influence on Cognitive Health in High Cardiovascular Risk Subjects (EBBINGHAUS) study(2), was used to evaluate cognitive function using the Cambridge Neuropsychological Test Automated Battery (CANTAB) in patients taking evolocumab. The primary endpoint was the score on the spatial working memory strategy index of executive function and secondary endpoints were the scores for working memory, episodic memory and psychomotor speed. A total of 1,204 patients were followed for a median of 19 months. There were no significant between-group differences in the primary and secondary endpoints (p<0.001 for noninferiority). There was no evidence of differences in cognitive tests by achieved nadir low-density lipoprotein cholesterol (LDL-C), even < 25 mg/dL
Within the broad group of patients with prior myocardial infarction (MI) in FOURIER, features were sought which would identify subsets that derive greater clinical risk reduction with evolocumab. In a multivariable adjusted model, 1) more recent MI, 2) multiple prior MIs and 3) residual multivessel coronary disease remained independent predictors of cardiovascular outcomes, with adjusted HRs for all P<0.001. This subset of “high risk patients” were at a 34-90 percent greater risk for major vascular events. In addition, these patients experience substantial relative risk reductions (21-30 percent) and absolute risk reductions (2.6-3.4 percent over three years) with intensive LDL-C lowering with evolocumab.(3)
13.2% of FOURIER patients had established peripheral arterial disease (PAD). Patients with PAD had an 81% higher risk of cardiovascular disease/MI/stroke compared with those without (p< 0.001). There was a significant reduction in cardiovascular disease/MI/stroke with evolocumab versus placebo among patients with PAD (9.5% vs. 13.0%, p=0.004) and those without (6.2% vs. 7.6%, p<0.001). The absolute risk reduction was greater among PAD patients due to the higher event rates. There was a significant reduction in the incidence of major adverse limb events with evolocumab (0.27% vs. 0.45%, p=0.0093), with greater net reductions among patients with PAD (1.5% vs. 2.4%). There appeared to be a monotonic relationship between reduction in LDL-C and reduction in adverse limb events (p=0.026). Further, evolocumab reduced the risk of major adverse limb events in all patients with consistent effects in those with and without known PAD. Benefits extend to PAD without prior MI or stroke with an ARR for major adverse cardiovascular events (MACE) or major adverse limb events (MALE) of 6.3% (number needed to treat (NNT) 16) at 2.5 years.(4)
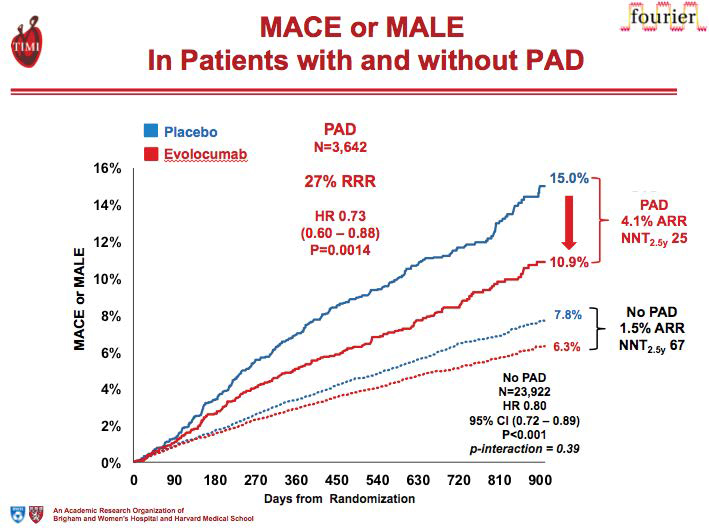
Figure 1. Graph Showing Major Adverse Cardiac Event and Major Adverse Limb Events with /without Peripheral Artery Disease.
In terms of maximal statin therapy, evolocumab safely decreased cardiovascular events in patients with stable ASCVD to a similar degree whether the baseline LDL-C was <70 or ≥70 mg/dL, and regardless of whether the background statin was maximal intensity or not.(5)
The FOURIER trial further investigated whether the efficacy of evolocumab is modified by baseline inflammatory risk.(6) The efficacy of evolocumab was stratified by baseline high-sensitivity C-reactive protein (hsCRP). A total of 7,981 (29%) patients had a baseline hsCRP < 1 mg/L, 11,177 (41%) had an hsCRP 1 to 3 mg/L, and 8,337 (30%) had a hsCRP > 3 mg/L. Median (interquartile range) baseline hsCRP was 1.8 (0.9–3.6) mg/L and levels were not altered by evolocumab. The relative risk reductions for the primary end point and key secondary end point with evolocumab were consistent across hsCRP strata . In summary 1) the relative benefit of evolocumab for decreasing risk of CV events was consistent irrespective of baseline hsCRP. 2) Patients with higher hsCRP had higher event rates and tended to experience greater absolute CV risk reduction with evolocumab. 3) CV event rates were independently associated with both LDL-C and hsCRP, even in pts with very low achieved LDL-C levels (< 20 mg/dL) Event rates were lowest in patients with the lowest hsCRP and LDL-C.High risk subgroups were also studied including diabetes.(7) At study baseline, 40 percent of patients had diabetes (n=11,031) and 60 percent did not have diabetes (n=16,533), of whom 10,344 had pre-diabetes and 6,189 had normoglycemia. In summary:1) Evolocumab was efficacious in ASCVD patients with and without diabetes with 57-60% decrease in LDL-C (p<0.001). 2) There was an 18-22% relative risk reduction in CVD/MI/stroke, and the benefit increases over time. 3) Given higher baseline risk, larger absolute risk reduction in CV events with evolocumab were seen in patients with diabetes (particularly coronary revascularization). Evolocumab showed no increased risk of diabetes, even in patients with prediabetes and no worsening of glycemia.
“The FOURIER trial showed interesting and exciting data in reference to subgroup evaluation.”
The thrombolysis in myocardial infarction (TIMI) risk score for Secondary Prevention (TRS 2°P)(8) has been used to identify patients who have the greatest potential for benefit from evolocumab. The 10-point integer-based scheme showed a strong graded relationship with the rate of CV death, MI or stroke and the individual components (p-trend<0.0001 for all). The TRS 2°P identifies high-risk patients with ASCVD who demonstrate a pattern of greater absolute risk reduction in major CV events with Evolocumab.
LDL lowering and “Ultra low LDL” has been an area of significant interest.
.jpg)
Summary of Subgroup Analysis of Fourier Trial and Evolocumab
(9) Among FOURIER patients, 31% had their LDL-C reduced to 20-49 mg/dL, 8% achieved an LDL-C level of 10-19 mg/dL, and 2% reached a remarkable LDL level of below 10 mg/dL. After a median follow-up of 26 months, the incidence of the study’s primary endpoint dropped by a statistically significant 15% in patients with an achieved LDL-C of 20-49 mg/dL, compared with patients whose 4-week LDL-C was at or above 100 mg/dL (primarily patients randomized to the study’s control arm), by 24% in all patients with LDL-C less than 20 mg/dL, and by 31% in the 2% of patients whose LDL-C levels fell below 10 mg/dL. These strikingly improved event rates at the lowest levels of LDL-C occurred with no signal of excess adverse events. There was a monotonic relationship between achieved LDL-C and major cardiovascular outcomes down to LDL-C concentrations of less than 8 mg/dL.
Subgroup analysis of Lp(a) in the FOURIER trial revealed that evolocumab significantly reduces Lp(a) concentration (median % change 26.9%).(10) Patients starting with higher Lp(a) levels appear to derive greater absolute benefit from PCSK9 inhibition. Patients who achieve lower levels of both LDL-C and Lp(a) have the lowest subsequent risk of CV events (6.5% vs 9.43%). The role of Lp(a) with PCSK9 inhibition treated patients has been further clarified by new clinical trial data from the ODYSSEY OUTCOMES trial showing that 1) baseline Lp(a) is an independent predictor of MACE and nonfatal MI among patients with recent acute coronary syndrome (ACS), independent of treatment with alirocumab and baseline LDL-C, and 2) Lp(a) lowering by alirocumab is associated with a reduced risk of MACE and non-fatal MI, independent of baseline LDL-C and concurrent LDL-C lowering.(11)
In conclusion, the FOURIER trial showed interesting and exciting data in reference to subgroup evaluation. Lowering LDL-C to unprecedented low levels is particularly effective in the highest patients including those with multivessel disease, PAD, and diabetes, with no excess in adverse events, and no lower limit to LDL-C.
Disclosure statement: Dr. Pokrywka received honoraria from Amarin, Sanofi, Regeneron, and Kowa. Dr. Patel received honoraria from Novaris, Jansen and Janssen, Amgen, Amarin, Kowa, Boston and Akcea





.jpg)
.png)












