Here we describe a case study of a 50- year-old physically active male wit h no significant past medical history wh o presented initially with fatigue an d shortness of breath while playing tenni s and was found to have new findings of an inferior wall MI on EKG. He wa s referred to Cardiology for a nuclea r stress study which showed a moderat e sized, severe fixed inferior wall defect. He had well-preserved functional exercis e capacity during the study. His lipid panel exhibited an LDL of 118 m g/dL, HDL 51 m g/dL, total cholesterol 184 m g/dL, and triglycerides 76 m g/dL. He did not have a personal history of tobacco use, diabetes , hypertension, hyperlipidemia, or obesit y nor did he have a significant family histor y of premature vascular disease. Up on subsequent coronary angiography, he wa s found to have subtotal occlusion of th e right coronary artery necessitating a drug-eluting stent, as well as 40% blocka ge of the left anterior descending artery an d preserved left ventricular ejection fraction . With further studies, this relatively l ow risk patient was ultimately found to have an elevated lipoprotein(a) level of 121nmol/L. Given the substantial burden of cardiovascular disease, understanding of risk factors and targets for intervention is paramount. Lipoprotein(a), Lp(a), is an emerging biomarker whose role in cardiac risk stratification is rapidly growing.(1)
Pathophysiology of Lp(a)
Lp(a) is a lipoprotein comprised of a LDL particle containing one molecule of apolipoprotein B-100 attached to apolipoprotein A by a disulfide bond. The mechanism by which Lp(a) results in increased atherogenesis is thought to be through two different pathways, via atherosclerosis and thrombosis. Macrophages have the ability to uptake Lp(a), leading to foam cell formation and increased delivery of cholesterol to the subendothelial space.(2) Lp(a) is homologous to plasminogen and can inhibit tissue plasminogen activator-mediated fibrinolysis, thus preventing thrombolysis.(2)
Lp(a) and Cardiovascular Risk
The levels of Lp(a) are predominantly genetically determined and expressed at stable levels by around age 5 and persist through adulthood.(3) These levels remain without much alteration other than in periods of acute inflammation. They are generally higher in African Americans. The global prevalence of Lp(a) levels over 50 mg/dL is around 1.8 billion individuals, per the 2018 report by National Heart, Lung, and Blood Institute.(2) Lp(a) is often measured in either mg/dL or nmol/L. This does make standardization of measurement difficult as it is not possible to convert between the two units. Given the varied molecular weights of Lp(a) isoforms, nmol/L takes into account particle concentration preventing significant over- or underestimation versus mass concentration when measured in mg/dL.(1) High Lp(a) levels are associated with elevated risk of atherosclerotic cardiovascular disease (ASCVD).(3) The risk is independent of levels of traditional lipoproteins, i.e. LDL-C. Levels over 20-30mg/dL have a greater risk of contributing to the development of coronary artery disease and the associated risk increases proportional to the Lp(a) level.(2) According to the INTERHEART trial, Lp(a) levels over 50 mg/dL conferred a greater risk of MI varying across ethnicities, but particularly evident in South Asians. (4) Interestingly, elevated lipoprotein levels may have a more potent risk in females compared to males, although this remains controversial and requires further evaluation.(2)
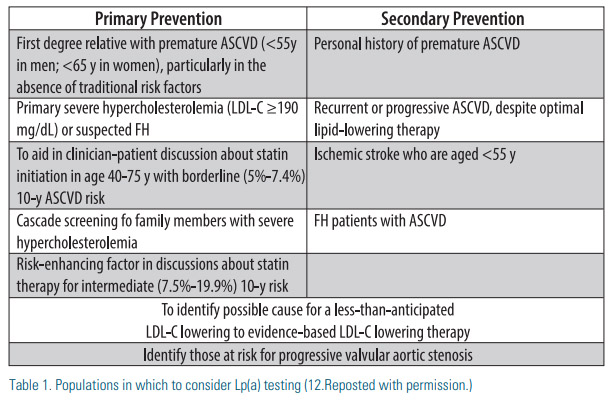
Increased Lp(a) levels appear to predict a higher likelihood of in-stent coronary restenosis.(5) In a subset of patients amongst an Asian population with angina who received elective percutaneous coronary intervention with drug-eluting stent, those with Lp(a) levels greater than 50 mg/dL had a greater incidence of binary restenosis and an overall poor 3-year outcome.(5) Elevated Lp(a) is also associated with increased risk of calcific aortic stenosis, with levels greater than 90mg/dL associated with a threefold increased risk.(6) Familial hypercholesterolemia patients are another group where Lp(a) levels over 50mg/dL portends a higher risk of CVD independent of LDL-C levels.(7) Patients that may benefit from Lp(a) testing include patients with: 1) intermediate risk for primary prevention when considering initiation of statin therapy; 2) very high risk secondary prevention for consideration of PCSK9 inhibitors; 3) personal or family history of premature ASCVD (especially in the absence of traditional risk factors); 4) familial hypercholesterolemia. See Table 1.
Therapies for Lp(a) Reduction
There is currently no FDA approved therapy for the specific purpose of lowering Lp(a). Traditional lipid-lowering therapies, such as statins, fibrates, and ezetimibe have not been shown to lower Lp(a). Niacin was shown to reduce Lp(a) levels by about 23% although it did not provide a noticeable benefit in reducing MACE (major adverse cardiovascular events) in the “Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides” (AIM-HIGH) trial.(8) LDL Apheresis has been shown to have effective results in lowering Lp(a) levels, although its utility is limited secondary to logistical concerns, such as limited availability of apheresis centers, cost of therapy, intravenous access, interruption of normal activity to perform procedures regularly.(3) The “Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk” (FOURIER) trial showed that the PCSK9 inhibitor evolocumab reduced Lp(a) levels by approximately 27%.(9) A pre-specified analysis of the ODYSSEY OUTCOMES trial showed that alirocumab reduced Lp(a) levels by a median of 23% in patients with recent acute coronary syndrome within the past 12 months.(10) Lp(a) lowering by alirocumab resulted in reduction in major adverse cardiovascular events, independent of LDL lowering. This suggests that Lp(a) should be an independent target of therapy after acute coronary syndrome. Looking to the future, AKCEA apo(a)-LRx, an antisense oligonucleotide that specifically targets apo(a) production, has been shown to reduce Lp(a) levels by up to 80% and is currently undergoing phase 3 clinical trials to assess its impact on cardiovascular outcomes.(11)
Conclusion
Lp(a) is an important biomarker with an expanding role in cardiovascular risk stratification. More research is needed in its effect on different cardiovascular patient populations as well as specific Lp(a) targeted therapies.
Disclosure statement:
Dr. Singh has no financial disclosures to report. Dr. Meng has no financial disclosures to report.
References:
1. Wilson, D. P., Jacobson, T. A., Jones, P. H., Koschinsky, M. L., Mcneal, C. J., Nordestgaard, B. G., & Orringer, C. E. (2019). Use of Lipoprotein(a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. Journal of Clinical Lipidology, 13(3), 374-392.
2. Maranhão, R. C., Carvalho, P. O., Strunz, C. C., & Pileggi, F. (2014). Lipoprotein (a): structure, pathophysiology and clinical implications. Arquivos brasileiros de cardiologia, 103(1), 76–84. https://doi.org/10.5935/abc.20140101
3. Scheel, P., Meyer, J., Blumenthal, R., & Martin, S. (2019, July 2). Lipoprotein(a) in Clinical Practice. Retrieved March 15, 2020, from http://www.acc.org/latest-in-cardiology/articles/2019/07/02/08/05/ lipoproteina-in-clinical-practice
4. Enas, E. A., Varkey, B., Dharmarajan, T., Pare, G., & Bahl, V. K. (2019). Lipoprotein(a): An underrecognized genetic risk factor for malignant coronary artery disease in young Indians. Indian Heart Journal, 71(3), 184–198. doi: 10.1016/j.ihj.2019.04.007
5. Park SH, et al. Impact of high lipoprotein(a) levels on in-stent restenosis and long-term clinical outcomes of angina pectoris patients undergoing percutaneous coronary intervention with drug-eluting stents in Asian population. Clin Exp Pharmacol Physiol. 2015 Jun;42(6):588-95. doi: 10.1111/1440-1681.12396. 6.
6. Zheng KH, Tsimikas S, Pawade T, et al. Lipoprotein(a) and oxidized phospholipids promote valve calcification in patients with aortic stenosis. J Am Coll Cardiol 2019;73:2150–62.
7. Alonso, R., Andres, E., Mata, N., Fuentes-Jimenez, F., & Badimon, L., et. al (2014). Lipoprotein(a) Levels in Familial Hypercholesterolemia: An Important Predictor of Cardiovascular Disease Independent of the Type of LDL Receptor Mutation. Journal of the American College of Cardiology, 63(19), 1982–1989.
8. AIM-HIGH Investigators (2011). Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. New England Journal of Medicine, 365(24), 2255–2267. doi: 10.1056/ nejmoa1107579
9. O’Donoghue, M., Fazio, S., Giugliano, R., Stroes, E., & Kanevsky, E., et. al (2018). Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk Insights From the FOURIER Trial. Circulation, 139(12).
10. Bittner, V., Szarek, M., Aylward, P., Bhatt, D., & Diaz, R., et. al (2020). Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome. Journal of the American College of Cardiology, 75(2), 133–144.
11. Tsimikas S, et al. Lipoprotein(a) Reduction in Persons with Cardiovascular Disease. N Eng J Med. 2020 Jan 16;382(3):244-255.
12. Lipoprotein(a) in Clinical Practice by Scheel P, Meyer J, Blumenthal RS and Martin SS. The American College of Cardiology, 2020, http://www.acc.org/latest-in-cardiology/articles/2019/07/02/08/05/ lipoproteina-in-clinical-practice. © 2020 by the American College of Cardiology Foundation. Reposted with permission.





.jpg)
.png)












