We all see patients in our practice who either have complaints of muscle aches after starting HMG-Co-A Redtucase inhibitors (statins) or have been referred by other health care practitioners for management of dyslipidemia in the presence of suggested diagnosis of statin myopathy. A careful differential diagnosis should take into account many factors:
 1) The cause of the muscle pain.
1) The cause of the muscle pain.
2) The timing of the muscle pain with respect to statin initiation.
3) Confirming laboratory tests along with physical examination.
4) The dose and specific statin used.
5) The pharmacokinetics of the statin.
6) Other concomitant medications.
7) Pharmacodynamics with respect to statin metabolism which may be altered by genetic mutations in certain enzyme systems.
8) Other clinical conditions or implicating factors.
The following article reviews the pharmacokinetic and pharmacodyamic parameters of the statins with a focus on the evidence and clinical aspects of the management of the patient with statin myopathy.
Irrespective of statin use, other causes of myopathy have been noted. Many antibiotics along with cocaine or other illicit drugs have been associated with a focal myopathy. Colchicine, amiodarone, cyclosporine, glucocorticoids, cimetidine along with statins have been implicated in diffuse myopathy. Extreme physical activity has also been linked to severe muscle pain. A careful history, physical and laboratory data is essential to classification of a muscle problem.1-3
Useful laboratory tests include a basic metabolic panel to determine renal function and acid-base or electrolyte abnormalities and a creatinine phosphokinase (CPK) to categorize the degree of muscle damage. Serum and/or urine myoglobin may be helpful in the diagnosis of rhabdomyolysis.4-6
.png) Factors that pre-dispose the patient to muscle diseases (Table 1) should be taken into account. Common among these are family history of statin muscle pain, recent vigorous exercise and gender. The small frame, elderly female may respond more rigorously to small "pixie-dust" doses of statins than their male counterparts and require appropriate dose adjustments. Hypothyroidsim should be ruled out with thyroid function testing. Polypharmacy and multiple providers put patients at risk for drug interactions. A complete family history is must also be part of any comprehensive evaluation. Singlenucleotide polymorphisms (SNPs) impacting statin associated metabolic pathways have been implicated in the pathogenesis of statin myopathy.3,12
Factors that pre-dispose the patient to muscle diseases (Table 1) should be taken into account. Common among these are family history of statin muscle pain, recent vigorous exercise and gender. The small frame, elderly female may respond more rigorously to small "pixie-dust" doses of statins than their male counterparts and require appropriate dose adjustments. Hypothyroidsim should be ruled out with thyroid function testing. Polypharmacy and multiple providers put patients at risk for drug interactions. A complete family history is must also be part of any comprehensive evaluation. Singlenucleotide polymorphisms (SNPs) impacting statin associated metabolic pathways have been implicated in the pathogenesis of statin myopathy.3,12
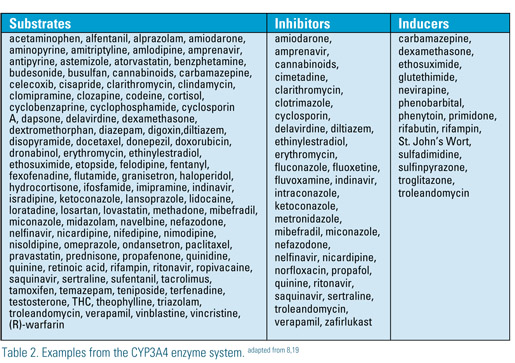 There are more than 30 different enzyme systems and multiple families of enzymes associated with liver metabolism. Commonly associated with statin interactions are CYP 2C9 and CYP3A4. Additionally, aging is associated with a 20- 30% reduction in hepatic size and a 20-50% reduction in hepatic blood flow. Despite this, there is conflicting information regarding the impact of aging on hepatic microsomal enzyme activity. Some patients with active liver disease or nonalcoholic steatohepatitis (NASH) may have altered enzyme activity. There is, however, general consensus that these patients should be treated with statins due to cardiovascular risk associated with this condition. A recent FDA ruling no longer recommends that liver done routinely before starting statin therapy, certain patients such as those with active liver disease and NASH may require additional monitoring.11,13,18,21
There are more than 30 different enzyme systems and multiple families of enzymes associated with liver metabolism. Commonly associated with statin interactions are CYP 2C9 and CYP3A4. Additionally, aging is associated with a 20- 30% reduction in hepatic size and a 20-50% reduction in hepatic blood flow. Despite this, there is conflicting information regarding the impact of aging on hepatic microsomal enzyme activity. Some patients with active liver disease or nonalcoholic steatohepatitis (NASH) may have altered enzyme activity. There is, however, general consensus that these patients should be treated with statins due to cardiovascular risk associated with this condition. A recent FDA ruling no longer recommends that liver done routinely before starting statin therapy, certain patients such as those with active liver disease and NASH may require additional monitoring.11,13,18,21
The issue surrounding the drug-drug interactions appears to be related to the serum and or intra-cellular levels of statins. When doses are increased higher levels of the statin acid result and muscle toxicity can occur. This first became evident in the late 1990s when an attempt at approving a 160mg dose of simvastatin was abandoned due to higher levels of myotoxicity. In August 2001, cerivastatin was recalled due to the gemfibrozil-cerivastatin interaction which produced very high serum concentrations of the parent drug and some of its metabolites.19
Recently the FDA, following an analysis of the SEARCH trial, capped the maximal dose of simvastatin at 40mg daily due to the burden of myopathy and rhabdomyolysis at the higher dose of the drug. The latter problem was highest during the first year of treatment. Similarly, in February 2012 the FDA announced similar new labeling for lovastatin. Table 3 summarizes new FDA labeling for lovastatin and simvastatin.20,21
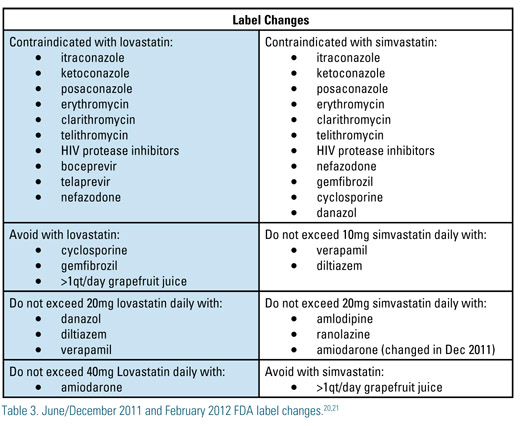 The new hepatitis C drugs, boceprivir and telaprivir, may inhibit the metabolism of simvastatin through CYP 3A4. Grapefruit juice in large quantities inhibits CYP3A4 and hence simvastatin, lovastatin and atorvastatin. Once inhibited the ability of the enzyme takes up to 24 hours to rebound to pre-inhibitory concentrations after withdrawal of the grapefruit juice. HAART regimen with newer or older protease inhibitors(PI) Amprenavir, saquinavir, lopinavir, duranavir and other protease inhibitors increase the risk for atorvastatin, simvastatin and lovastatin myopathy. Changing the statin or changing the PI to an alternative agent may be required. Amiodarone is metabolized through CYP3A4 and CYP2C8 and dose adjustments with some statins are required when with this agent. The FDA arbitrarily lowered the simvastatin dose cap to 10mg when taken with amiodarone. This was redacted in December 2011 to a maximum simvastatin dose of 20mg due to lack of clinical trial or pharmacokinetic data to support the lower dose.20,21
The new hepatitis C drugs, boceprivir and telaprivir, may inhibit the metabolism of simvastatin through CYP 3A4. Grapefruit juice in large quantities inhibits CYP3A4 and hence simvastatin, lovastatin and atorvastatin. Once inhibited the ability of the enzyme takes up to 24 hours to rebound to pre-inhibitory concentrations after withdrawal of the grapefruit juice. HAART regimen with newer or older protease inhibitors(PI) Amprenavir, saquinavir, lopinavir, duranavir and other protease inhibitors increase the risk for atorvastatin, simvastatin and lovastatin myopathy. Changing the statin or changing the PI to an alternative agent may be required. Amiodarone is metabolized through CYP3A4 and CYP2C8 and dose adjustments with some statins are required when with this agent. The FDA arbitrarily lowered the simvastatin dose cap to 10mg when taken with amiodarone. This was redacted in December 2011 to a maximum simvastatin dose of 20mg due to lack of clinical trial or pharmacokinetic data to support the lower dose.20,21
The inhibition of statin metabolism is not limited to the CYP3A4 enzyme system. Statins and non-statins, such as ezetimibe and gemfibrozil are affected by interacting agents. Inhibitors of CYP2C9 and CYP3A4 will likely increase the area under the concentration-time curve (AUC) as well as peak plasma concentrations of many statins. These higher drug levels are thought to be directly related to increased incidence statin adverse reactions. Inducers the activity of these enzymes will increase the catabolism of the drug rendering it less effective.3,18,19,22
Uridine diphosphate glucuronosyltransferase (UGT) has been previously implicated in the gemfibrozil–cerivastatin interaction. Ezetimibe undergoes rapid glucuronidation in intestinal mucosa cells by UGT1A1 and UGT1A3 and to a lesser degree, UGT2B15. Both fenofibrate and gemfibrozil can increase levels of ezetimibe due to their interaction with UGT enzyme family. The latter is not thought to be clinically significant.4,22
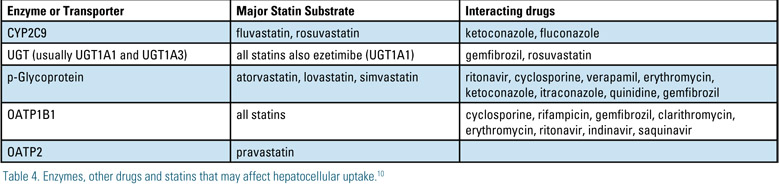 In addition to liver enzymes, there are a number of transporters responsible for hepatic uptake and clearance of statins. Table 3 lists enzymes that have been implicated in statin myopathy and rhabdomyolysis. P-Glycoprotein (pGp) is an ATP dependent efflux pump member of the ABCB1 transport protein. Its biologic function is to prevent toxins from being absorbed through the GI tract and to actively excrete toxins in the liver and kidney. Drugs that interact with pGp can either inhibit or induce the activity of this protein. This alters transport of the substrate. Examples of common pGp interactions are noted on Table 5.22
In addition to liver enzymes, there are a number of transporters responsible for hepatic uptake and clearance of statins. Table 3 lists enzymes that have been implicated in statin myopathy and rhabdomyolysis. P-Glycoprotein (pGp) is an ATP dependent efflux pump member of the ABCB1 transport protein. Its biologic function is to prevent toxins from being absorbed through the GI tract and to actively excrete toxins in the liver and kidney. Drugs that interact with pGp can either inhibit or induce the activity of this protein. This alters transport of the substrate. Examples of common pGp interactions are noted on Table 5.22
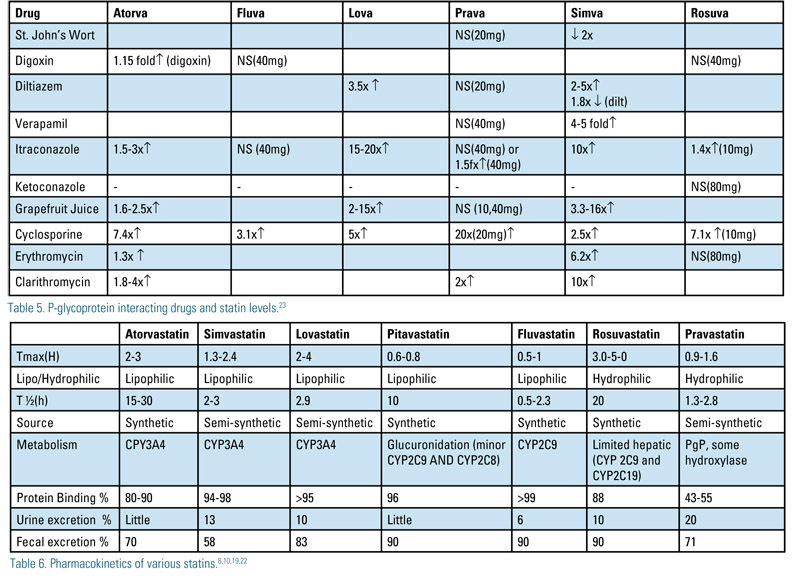 PGp interactions are mostly associated with CYP3A4 metabolized drugs. Fluvastatin and pravastatin do not significantly inhibit pGp transport. Rosuvastatin has not been shown to be a substrate or inhibitor of pGp or CYP3A4, yet when co-administered with itraconazole or cyclosporine serum concentrations increase. An alternative transporter, such as OATP2 may be responsible for the rosuvastatin-itraconazole interaction. Table 5 shows the effect of commonly given drugs (p-Glycoprotein substrates, inducers and inhibitors) and their effect on statin levels.23
PGp interactions are mostly associated with CYP3A4 metabolized drugs. Fluvastatin and pravastatin do not significantly inhibit pGp transport. Rosuvastatin has not been shown to be a substrate or inhibitor of pGp or CYP3A4, yet when co-administered with itraconazole or cyclosporine serum concentrations increase. An alternative transporter, such as OATP2 may be responsible for the rosuvastatin-itraconazole interaction. Table 5 shows the effect of commonly given drugs (p-Glycoprotein substrates, inducers and inhibitors) and their effect on statin levels.23
Polymorphisms in additional transport systems may offer an alternative explanation for statin induced myopathy. OATP1B1 is an uptake transporter located on the hepatocyte and is responsible for transport of statins from the portal circulation to the hepatocyte. In the recent SEARCH trial it was noted that more than 60% of the myopathies were attributed to a noted gene variant in this enzyme system. This SLCO1B1 521T>C polymorphism may be associated with increased of some statins such as simvastatin, but not pravastatin.
When approaching statin-drug interactions, the pharmacokinetics of statins need to be considered (Table 6). Lipophilicity appears to have little role in predicting statin intolerance as does half-life. Synthetic statins may be more tolerable than semisynthetic statins.
Drug-drug interactions, dosing, patient characteristics, pharmacodynamics and pharmacogenomics are all players in the picture of statin induced muscle disorders. In many patients statin metabolism is altered producing higher serum drug levels resulting in symptoms of myalgia or myopathy. The science of statin metabolism continues to expand, with the hope that someday a home monitoring test will be available to assist the patient in profiling which drug and or drug combination is most safe and efficacious.
Disclosure statement: Dr. Kellick has no relevant disclosures.





.jpg)
.png)











